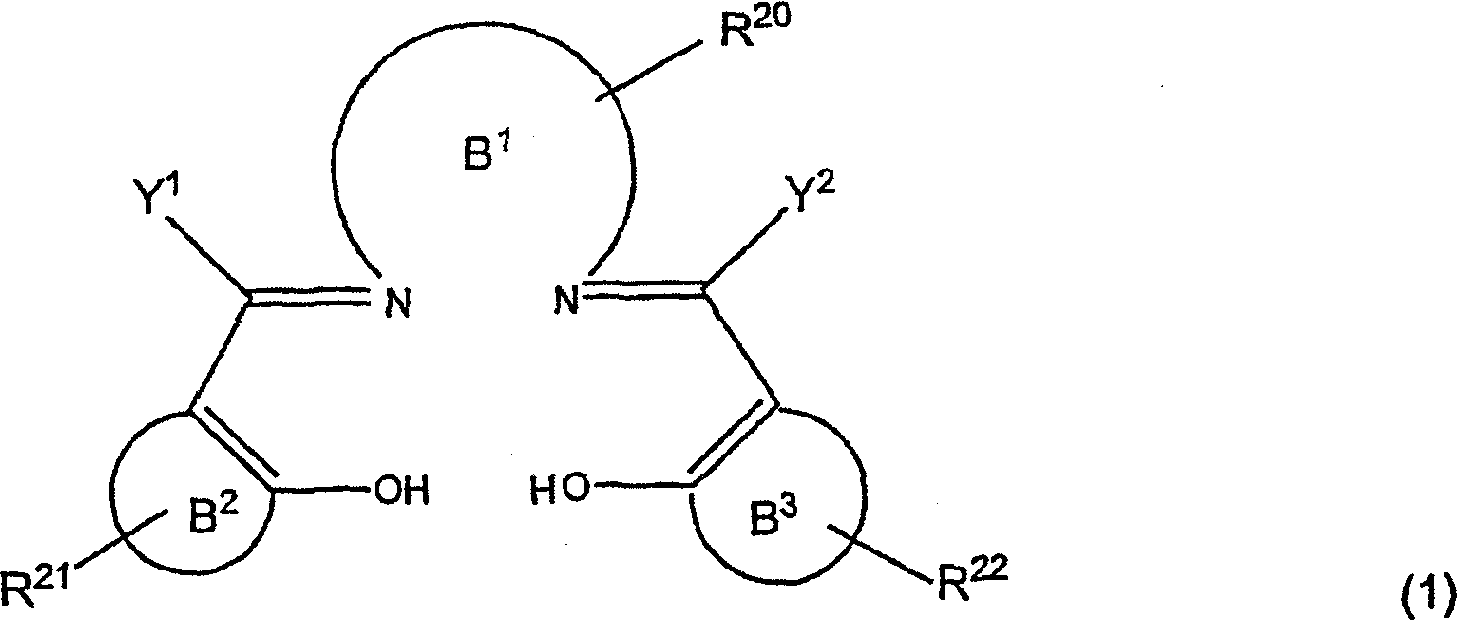
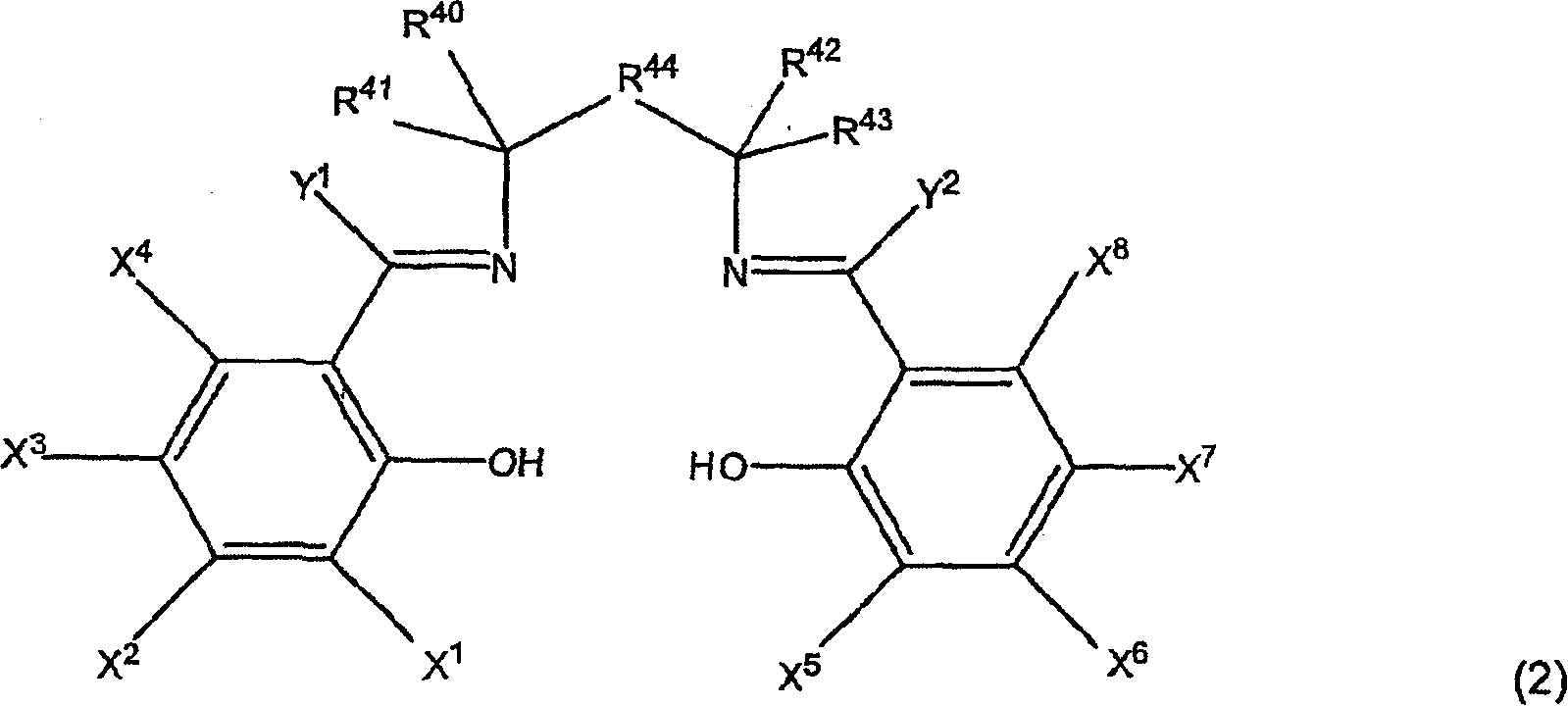
Active catalysts for stereoselective ring-opening reactions
A chiral catalyst, catalyst technology, applied in physical/chemical process catalysts, catalyst activation/preparation, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of high cost, time-consuming solvent removal, and increased cost And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] According to the following reaction scheme by making the chiral salen ligand, Co(OAc)·4H 2 The reaction of O, acetic acid and air in methanol produces an active catalyst:
[0167]
[0168] (R,R)-Jacobsen ligand 1 (82 g, 0.15 mol, 1.0 equiv) was added to a 3-liter three-necked round-bottom flask equipped with a mechanical stirrer, a thermometer, and an air-bubbling cannula, and then heated at ambient temperature MeOH (750 mL) was added to the vessel. Glacial acetic acid (18 g, 0.3 mol, 2.0 equiv) was then added to the flask. Add Co(OAc) to the reaction vessel 2 4H 2 O (41.9 g, 0.165 mol, 1.1 equiv) and an additional 75 mL (about 10 volumes total) of MeOH were used for flushing. The contents of the flask were stirred under open air for 30 minutes until most of the ligand solids had dissolved. A flow of air was then drawn through the dark green / brown mixture using a house vacuum through a cannula open to atmosphere with stirring for 2 hours. The contents of the fl...
Embodiment 2
[0170] According to the following reaction scheme by making the chiral salen ligand, Co(OAc) 2 4H 2 O, acetic acid and air react in excess acetic acid to produce an active catalyst:
[0171]
[0172] Add (S, S)-Jacobsen ligand 3 (2.2kg) in the reaction vessel equipped with high-speed stirrer, thermometer and air bubbling intubation, then add cobalt acetate tetrahydrate ( 1.06kg). The reaction vessel was purged with nitrogen. 18.5 kg of acetic acid was added to the reactor over a period of about 0.5 hr at room temperature with stirring started. The contents of the reaction vessel were stirred at room temperature while bubbling compressed air through the mixture at a rate of about 750 liters air / kg ligand / hour for 3 hours. Exhaust air is passed through a sodium hydroxide scrubber. The composition of the contents was monitored every 30 minutes by TLC. When the reaction was complete (>95% conversion), the air sparging was stopped, the vessel was purged with nitrogen and the...
Embodiment 3
[0174] Catalyst stability was assessed by monitoring the activity of the catalyst in the hydrolytic kinetic resolution ("HKR") of racemic epichlorohydrin versus the length of time the isolated catalyst was stored in a closed container at ambient temperature.
[0175] To a 125 mL jacketed vessel equipped with an overhead mechanical stirrer and a thermometer was added the (R,R)-Co(III)salen ligand-acetate-methanol catalyst produced according to the procedure given in Example 1 above Complex (1.66 g, 2.5 mmol, 0.5 mole % relative to racemic epoxide). Racemic epichlorohydrin (46.7 g, 0.5 mol) was added to the vessel and the temperature of the mixture was brought to 5°C with temperature controlled circulating liquid. Water (6.75 g, 0.375 mol, 0.75 equiv to racemic epoxide) was added over 2 hours using a syringe pump. After the addition was complete, the reaction was monitored by chiral GC analysis until completion of the reaction was indicated by an enantiomeric balance of >99% ep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com