Composition and method for forming electroactive coating comprising conjugated heteroaromatic polymer, capacitor and antistatic object comprising the electroactive coating, and solid electrolytic capacitor and method for fabricating the same
An electroactive polymer, electroactive technology, applied in the fields of electroactive polymer solution or coating, composition forming the same, capacitors and their manufacture, and antistatic objects, can solve problems such as reduced stability of mixtures, Achieve the effect of long service life, easy chemical operation, and simple manufacturing equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Example 1: Polymerization of pure liquid of 2-bromo-3-(butylthio)thiophene (BBTT) catalyzed by trifluoroacetic acid (TFA)
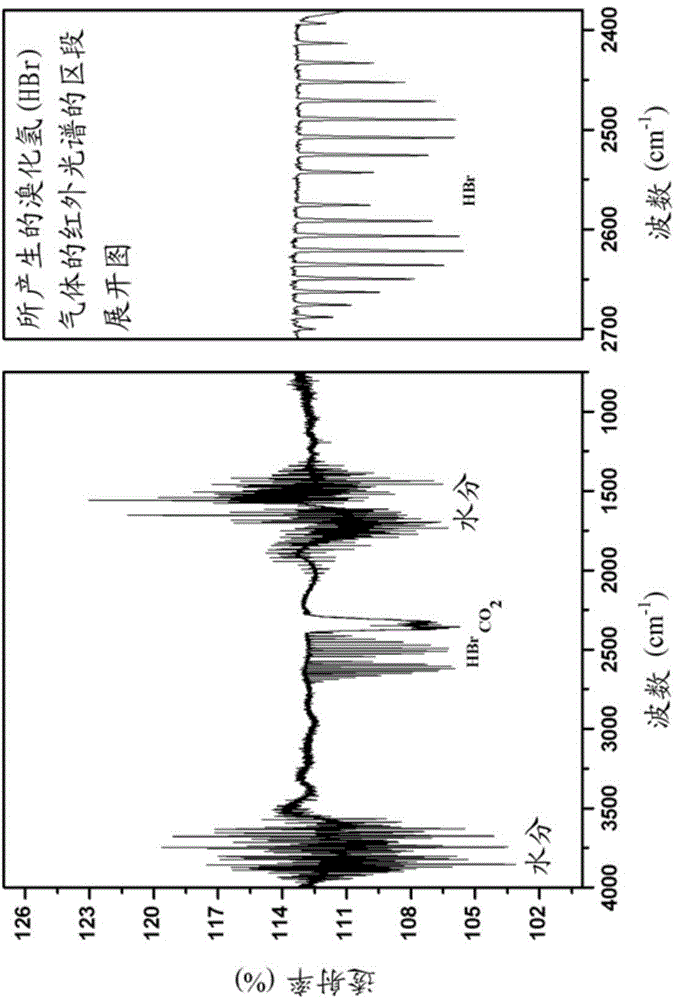
[0155] A pure liquid of 2-bromo-3-(butylthio)thiophene (1.00 g, 3.98 mmol) was mixed with 1.65 equivalents of trifluoroacetic acid (0.74 g, 6.49 millimoles) mixed. After the addition, the colorless liquid turned reddish brown first, and then turned into a dark blue colloid within 5 minutes, and produced a lot of smoke. The smoke produced was confirmed as hydrogen bromide gas by infrared spectroscopy, as figure 1 shown. This colloid cannot be stirred. After standing at room temperature for 24 hours, the thick blue colloid was dissolved in 20 ml of dichloromethane, and washed once with 10 ml of about 5% aqueous sodium hydroxide solution and twice with water (10 ml each time). The resulting red-brown solution was then dried over anhydrous magnesium sulfate, and then concentrated by a rotary evaporator to obtain a thick red-brown sticky solid in hi...
Embodiment 2
[0160] Example 2: Polymerization of BBTT catalyzed by TFA in toluene solution
[0161] A solution of 1.50 g (5.97 mmol) BBTT in 4.5 mL toluene was polymerized with 0.5 equivalents (0.34 g, 3.98 mmol) of trifluoroacetic acid in a single-neck round bottom flask under nitrogen at room temperature (25°C). The colorless solution turned reddish-brown immediately after the addition, and then turned into a dark blue viscous solution within 30 minutes, and produced a lot of hydrogen bromide fumes. After stirring at room temperature for about 2.5 hours, as in Example 1, this viscous solution was processed after completion of the reaction to obtain reddish-brown viscous poly[3-(butylthio)thiophene], and its yield was very high (1.05 g , close to 100%). Then identify the resulting product as in Example 1, find that its NMR and IR spectrum are substantially the same as those obtained in Example 1, and the ultraviolet light absorption maximum is at 453nm, M w It was found to be 1588, and ...
Embodiment 3~10
[0163] Examples 3-10: Polymerization of BBTT using different kinds and amounts of acids
[0164] The above-mentioned acid-catalyzed BBTT polymerization often can use different kinds of protonic acids, such as the aforementioned trifluoroacetic acid (TFA) and methanesulfonic acid (MSA), and the applicable range of acid consumption is wide (for example, about 0.1 to about 4 equivalents), and the results are arranged In Table 1. All the polymerization reactions shown in Table 1 can be carried out with high efficiency at room temperature, resulting in very high yields (nearly 100%). The polymerizations of Examples 3-6 all use BBTT pure liquid, which will turn into a dark blue solid within 5-10 minutes after being mixed with a specific amount of acid (see Table 1). Then the obtained solid matter was allowed to stand at room temperature for a period of reaction time shown in Table 1, and then treated as in Example 1.
[0165]The polymerizations of Examples 7-9 also started with BB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com