Pyrazolopyridines and methods of making and using the same
A technology for medicinal salts and compounds, applied in the fields of pyrazolopyridine and its preparation and use, can solve problems such as inability to bind to TGFβ receptors and lack of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
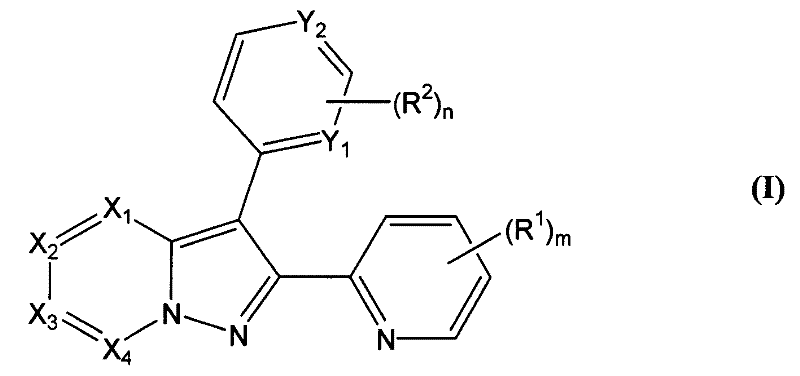
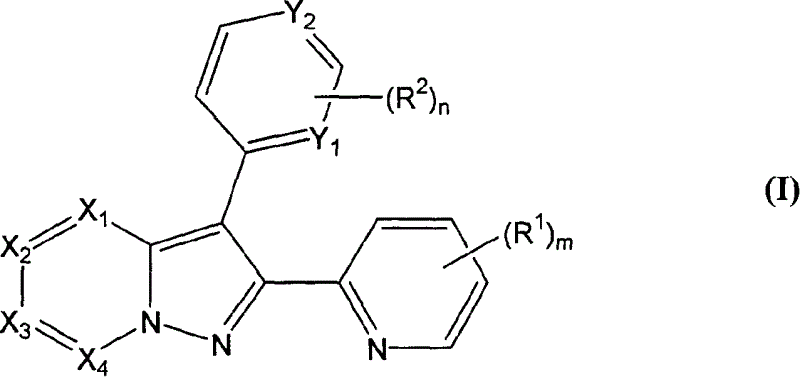
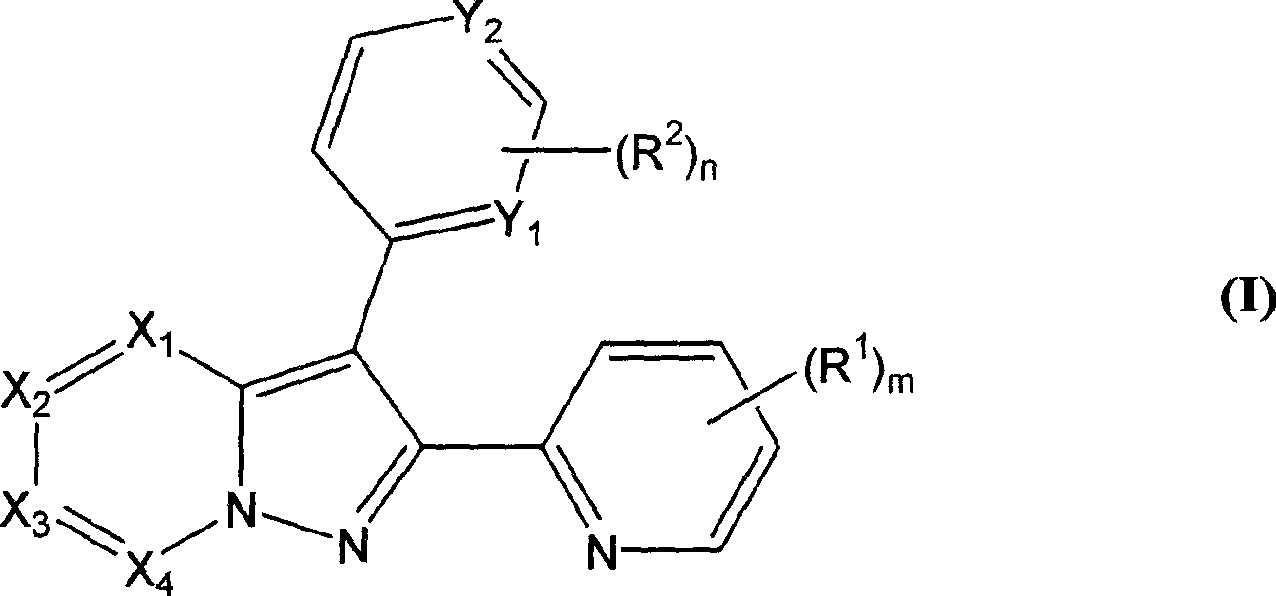
[0106] 4-[2-(6-Methyl-pyridin-2-yl)-pyrazolo[1,5-a]pyridin-3-yl]-pyrimidin-2-ylamine
[0107] The synthesis of the title compound is described in Sections (a)-(F) below.
[0108] (a) 2-Methyl-6-trimethylsilylethynyl-pyridine
[0109] Anhydrous triethylamine (45mL), PdCl 2 (PPh 3 ) 2 (0.48 mmol) and copper(I) iodide (1.45 mmol) were added to a solution of 6-bromo-2-picoline (48.2 mmol) in anhydrous DMF (110 mL). To the resulting orange solution was added dropwise (trimethylsilyl)acetylene (62.6 mmol). After stirring overnight at room temperature, the reaction was concentrated in vacuo and diluted with ether (100 mL), hexanes (100 mL) and water (100 mL). The emulsion was filtered through Celite pad and rinsed with ether. The separated organic phase was washed with water (1x), dried (MgSO 4 ) and concentrated in vacuo to afford 8.86 g of a dark brown oil identified as 2-methyl-6-trimethylsilylethynyl-pyridine. 1 H NMR (CDCl 3 , 400MHz): 0.24(s, 9H), 2.53(s, 3H), 7.06(d, ...
Embodiment 2
[0122] A cell-free assay to evaluate inhibition of TGFβ type I receptor autophosphorylation.
[0123] Determination of serine-threonine kinase activity of TGFβ type I receptors containing an N-terminal polyhistidine, TEV cleavage site marker, e.g., histidine-TGFβRI, as the autophosphorylation activity of the cytoplasmic domain of the receptor . The cytoplasmic kinase domain of the His-tagged receptor was purified from infected insect cell cultures using the Gibco-BRL FastBac HTb Baculovirus Population Expression System.
[0124] Assay buffer (50mM Hepes, 60mM NaCl, 1mM MgCl 2 , 2mM DTT, 5mM MnCl 2 , 2% glycerol and 0.015% Brij 35) in 20 μl 1.25 μCi 33 P-ATP / 25 μM ATP was added to a 96-well Nickel FlashPlate (NEN Life Science, Perkin Elmer). 10 [mu]l of the test compound of formula (I) in 5% DMSO solution was added to the FlashPlate. The assay was initiated by adding 20 μl of assay buffer containing 12.5 pmol of His-TGFβRI to each well. Plates were incubated at room tempe...
Embodiment 3
[0128] Cell-free Assay for Evaluating Inhibition of Activin Type I Receptor Kinase Activity
[0129] According to the method similar to the above-mentioned Example 2, replace His-TGFβRI with similar His-tagged Alk4 (His-Alk4), and the autophosphorylation activity of the test compound of formula (I) on activin type I receptor (Alk4) kinase can be determined inhibitory effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com