Copolymerization derivative of poly-asparagic acid and synthetic method
A technology of polyaspartic acid and synthesis method, which is applied in the field of polyaspartic acid copolymer derivatives and its synthesis, can solve the problems of no significant improvement in product scale inhibition performance, slow reaction speed, unstable performance, etc., and achieve shortening The effect of reaction time, fast reaction speed and stable product performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
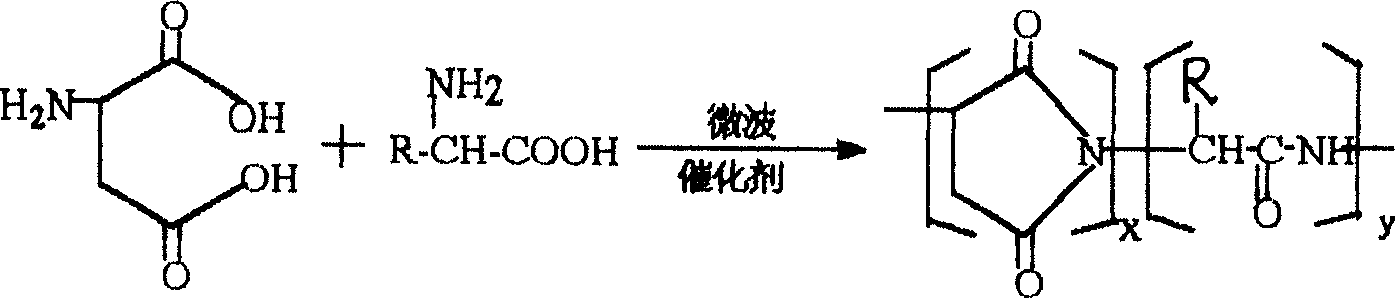
[0007] Embodiment 1: The structural formula of the polyaspartic acid copolymer derivative of the present embodiment is:
[0008] In the formula, R is H, (CH 3 ) 2 CH, (CH 3 ) 2 CHCH 2 、CH 3 CH 2 CH(CH 3 ), HOCH 2 、CH 3 CH(OH), HSCH 2 or HOOCCH 2 CH 2 , the range of y / (y+x) is 0.1-0.9, and the range of x+y is 7-70. The optimum range of y / (y+x) in this embodiment is 0.1-0.5, and the optimum range of x+y is 9-30.
specific Embodiment approach 2
[0009] Specific embodiment two: this embodiment synthesizes polyaspartic acid copolymer derivatives according to the following steps: with aspartic acid and For raw materials, where R is H, (CH 3 ) 2 CH, (CH 3 ) 2 CHCH 2 、CH 3 CH 2 CH(CH 3 ), HOCH 2 、CH 3 CH(OH), HSCH 2 or HOOCCH 2 CH 2 , aspartic acid and The stoichiometric number is 1.0-99.9, using microwave technology, under the conditions of microwave frequency 915±50MHz or 2450±50MHz, microwave power 100-30000W, add catalyst and solvent to the reactant, and radiate reaction for 1-40min. The precursor of the polyaspartic acid copolymer derivative can be obtained, and the polyaspartic acid copolymer derivative can be obtained by hydrolyzing the precursor, wherein the stoichiometric number of the catalyst and the reactant is 0.01 to 1.00, and the chemical ratio of the solvent and the reactant The metering number is 0.10~9.99, and the reaction equation is as follows:
[0010]
[0011] Aspartic acid Other am...
specific Embodiment approach 4
[0017] Specific embodiment four: Add aspartic acid and serine to a total of 0.18mol in a single-mouthed round bottom flask, aspartic acid and serine stoichiometric number is 7: 3, with NaH 2 PO 4 As a catalyst, the stoichiometric number of the catalyst and the raw material is 0.05, add 16ml of dimethylformamide, the microwave power is 720W, and when the microwave radiation time (Table 2) is different, fluffy products of different colors are obtained, and 32ml of 3mol / L NaOH is added Dissolve the product, adjust the pH of the solution to be neutral, filter, add the filtrate of the product to 250 ml of absolute ethanol, collect the precipitate, and dry it in vacuum at 70° C. to obtain a polyaspartic acid copolymer derivative.
[0018] radiation time
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com