Sulfonyl urea compounds and herbicidal activity thereof
A compound, sulfonylurea technology, applied in organic chemistry, animal repellents, plant growth regulators, etc., can solve problems such as large differences in crop sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
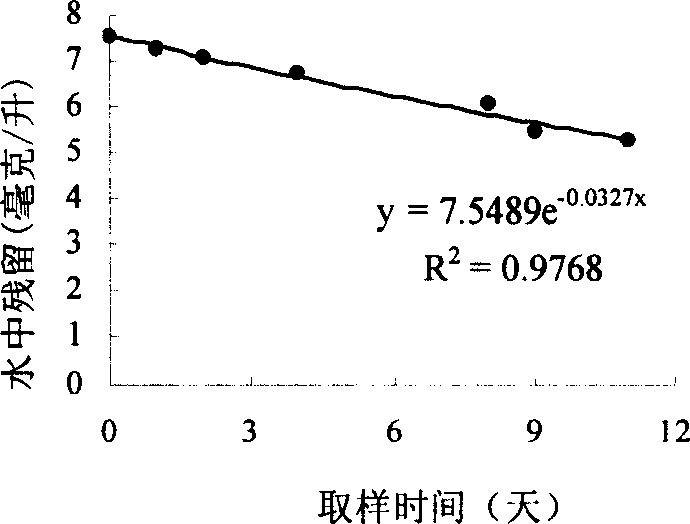
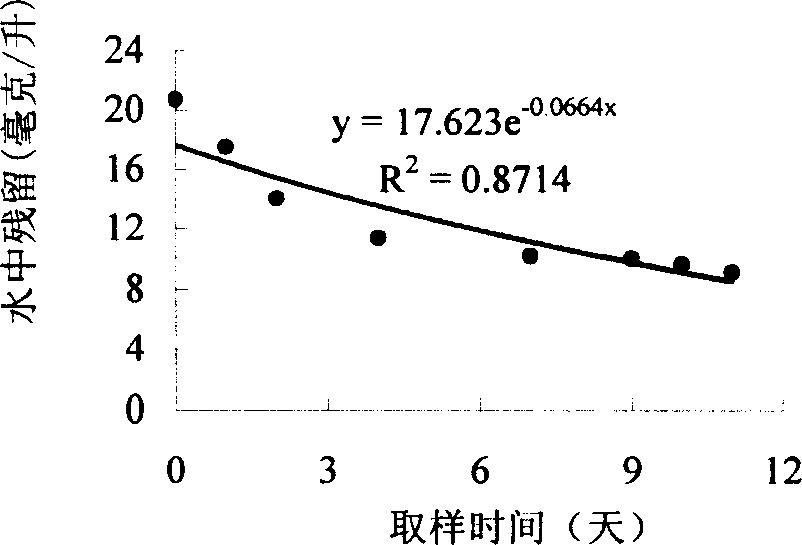
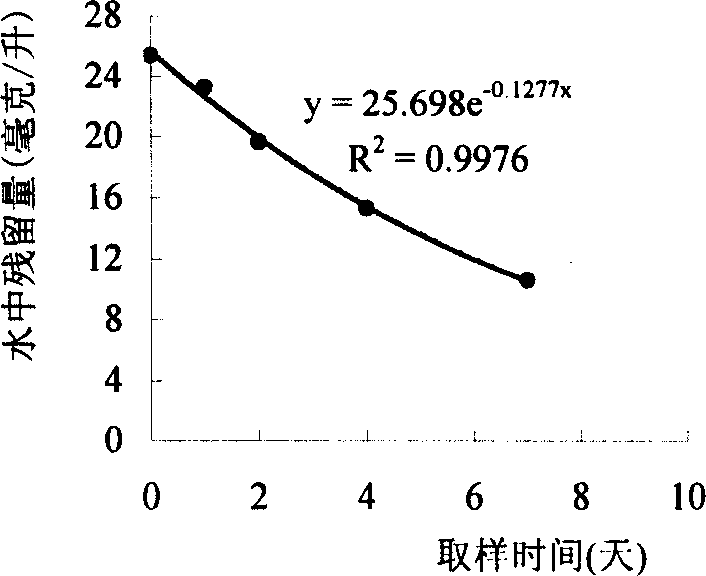
Image
Examples
Embodiment 1
[0062] Embodiment 1: the synthesis of 2-methoxy ester group 5-nitrobenzenesulfonamide
[0063] In the 100ml three-necked round-bottomed flask that is connected with tail gas absorption device, spherical condenser and thermometer, drop into 9.00 grams of 6-nitrosaccharin (Syntheses of saccharin and Cyclanate Derivatives Bearing Polymerizable Vinyl Group, Hiroyoshi K, et al.Bull.Chem. Soc.Jpn., 1982,55:3824-3827), add about 55ml of dry methanol, make it saturated with HCl gas, heat in an oil bath with stirring and slowly heat up to solvent reflux, continue to keep the temperature for 7 hours with HCl. The solvent methanol was distilled off under reduced pressure, and then saturated NaHCO 3 The pH value of the residual solid was adjusted to about 7.0, filtered, the filter residue was fully washed with water, and dried to obtain 6.45 g of white powder with a yield of 62.9% and a melting point of 196-198°C.
Embodiment 2
[0064] Embodiment 2: the synthesis of 2-methoxy ester group 5-nitrophenyl isocyanate
[0065] Add 3.9 grams (0.015mol) of 2-methoxy ester group 5-nitrobenzenesulfonamide, 30ml anhydrous toluene, 0.075mol in the 100ml three-neck round-bottomed flask equipped with drying tube, tail gas absorption reflux condenser and thermometer Oxalyl chloride, and add 0.25gDABCO (triethylenediamine) as a catalyst under stirring. Slowly raise the temperature to 60°C under stirring, keep at 60-65°C for 6 hours, then slowly raise the temperature to 90°C, react at 90-100°C for 18 hours, remove the solid by filtration, remove toluene and excess oxalyl chloride under reduced pressure The brown oily crude isocyanate was obtained, which was directly used in the next reaction without purification.
Embodiment 3
[0066] Embodiment 3: the synthesis of 6-aminosaccharin
[0067] Add 125ml of absolute ethanol, 5.0 grams (0.02mol) of 6-nitrosaccharin and 0.25 grams of 10% Pd / C in a 250ml three-necked flask, and introduce H for 4 hours under stirring. 2 , the insolubles were removed by filtration, and the filtrate was precipitated to obtain 3.93 g of a light yellow solid with a yield of 90.5% and a melting point of 286-289°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com