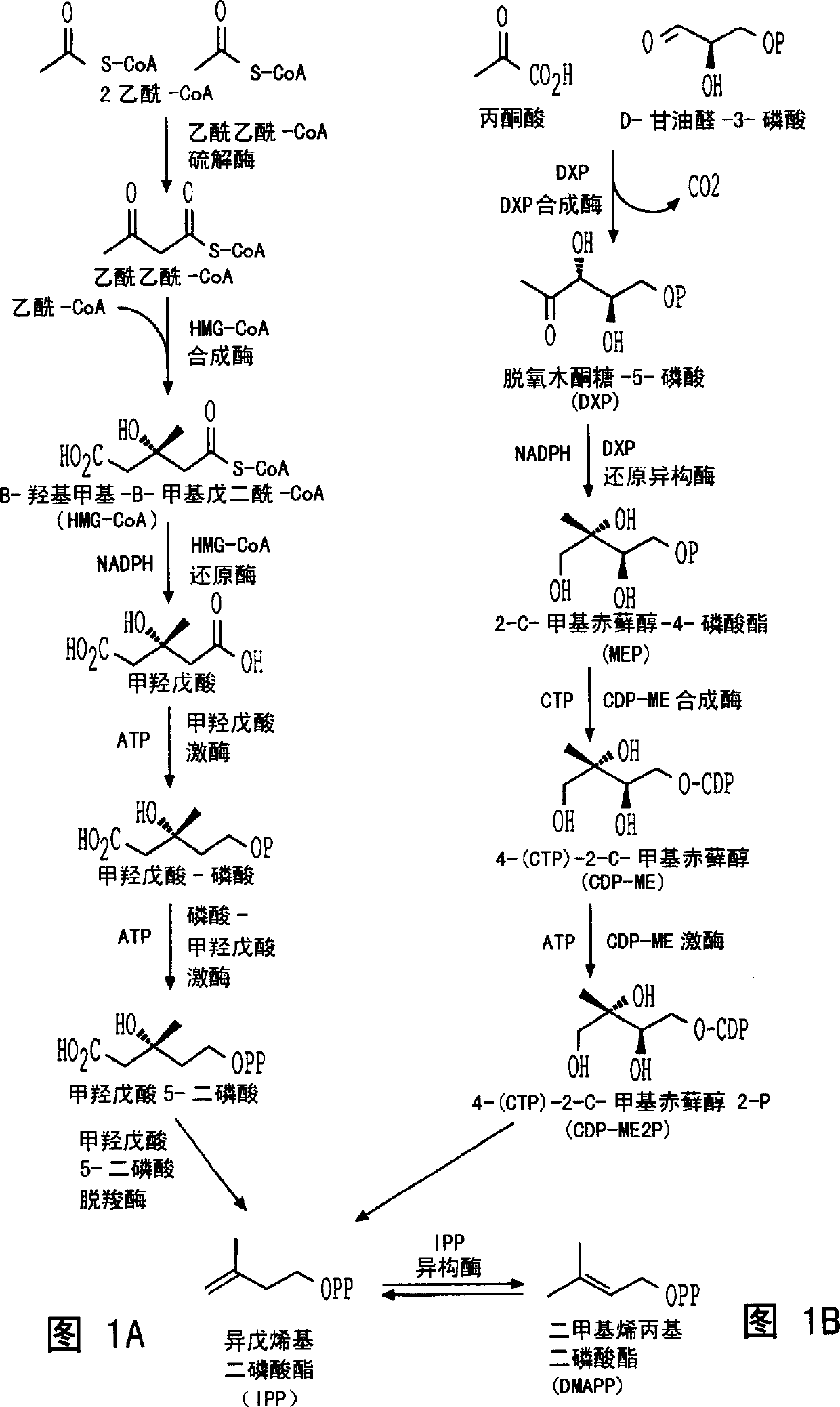
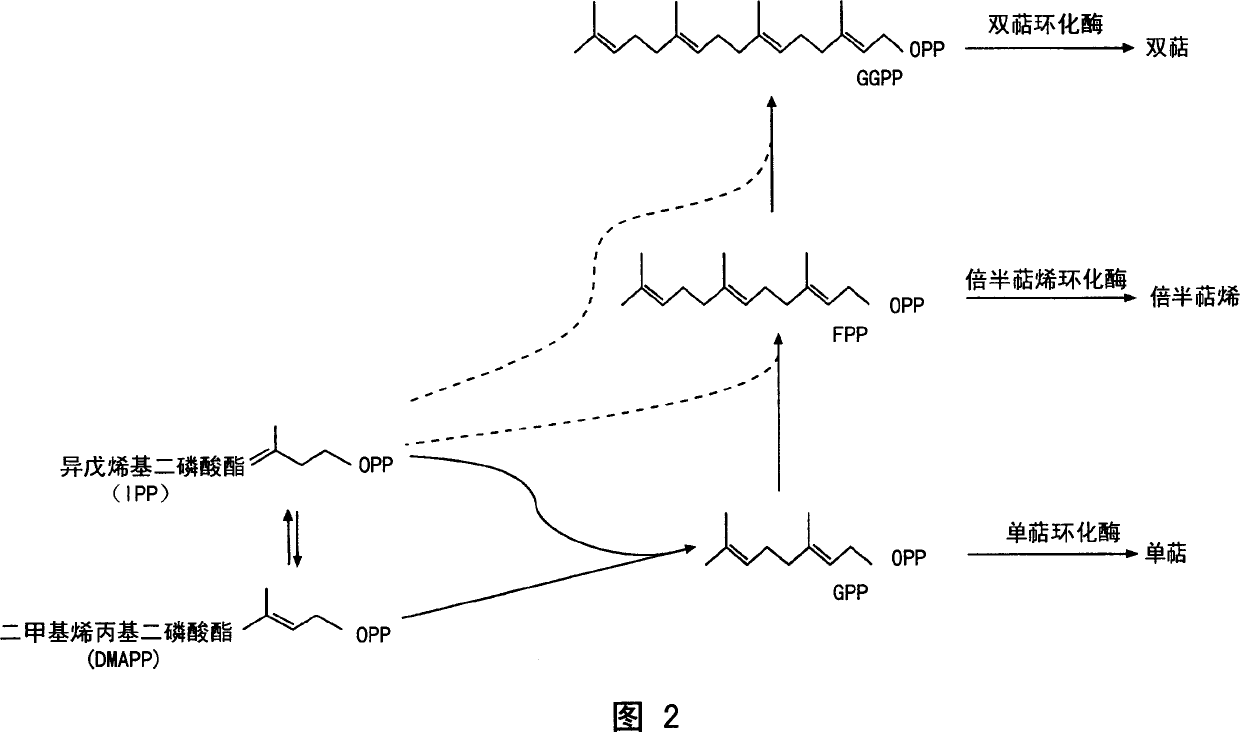
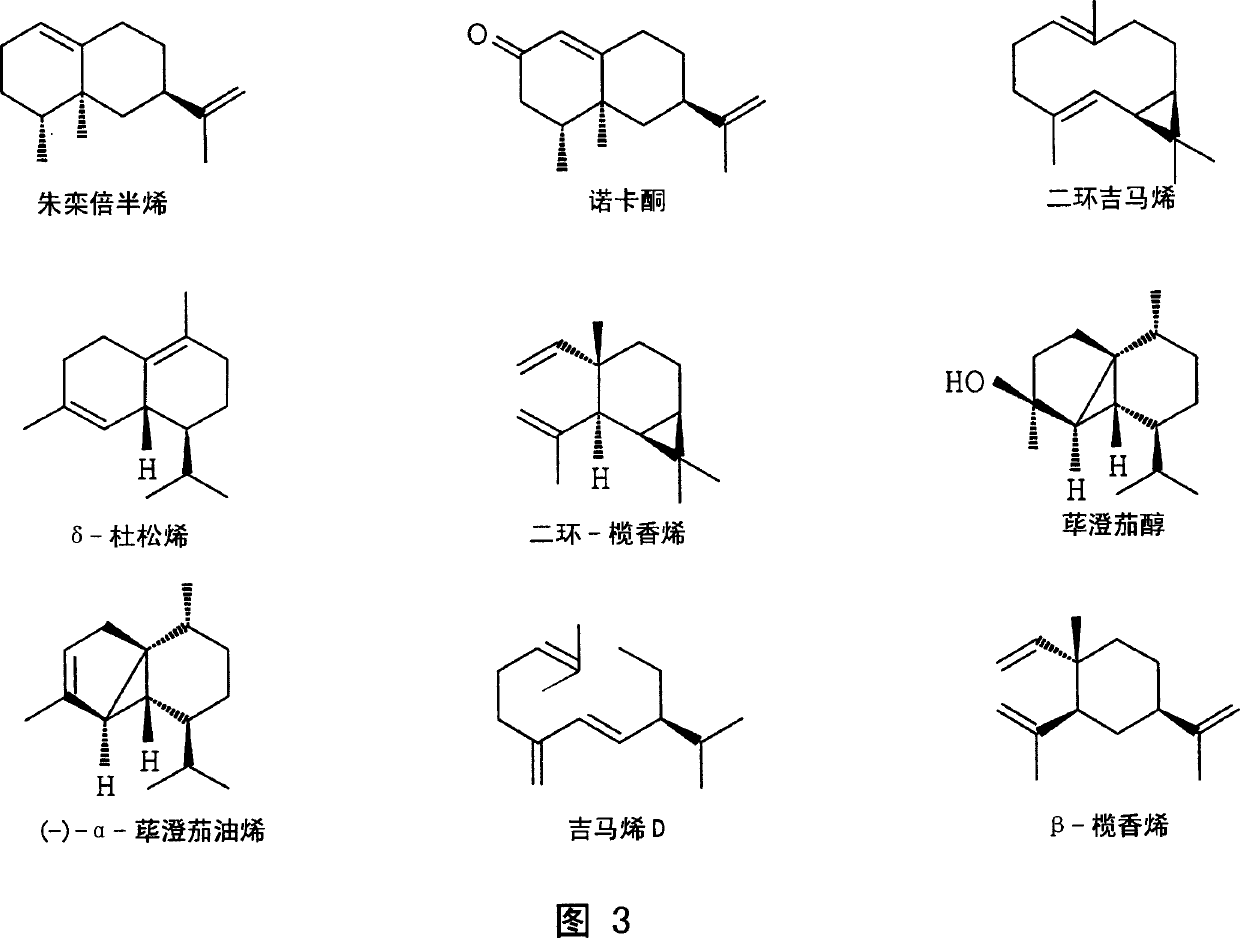
Sesquiterpene synthases and methods of use
A sesquiterpene and synthase technology, applied in the fields of sesquiterpene synthase and its preparation and use, can solve the problems of unacceptable chemical synthesis cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Isolation of sesquiterpene synthase cDNA by RT-PCR.
[0089] The deduced amino acid sequences of plant sesquiterpene synthases were aligned, conserved regions were identified and plant sesquiterpene synthase-specific oligonucleotides were designed. In order to obtain high sequence homology, the sequences were divided into two groups (Fig. 4). The first group included the sequences of the following enzymes: gimaene C synthase from tomato (Lycopersicon esculentum) cv.VFNT cherry (Colby et al, 1998), from Mentha x piperita (Crock et al, 1997 ), (E)-β-farnesene synthase from North American fir (Steele et al, 1998), and sesquiterpene synthesis from orange (GenBank accession number AF288465) Enzyme, 5-epi-aristolocene synthase from tobacco (Facchini and Chappell, 1992) and pepper (Back et al, 1998), vetispiradiene synthase from potato and Hyoscyamus muticus (Back and Chappel, 1995) . The second group included the sequences of the following enzymes: (+)-delta-jun...
Embodiment 2
[0095] Example 2: Isolation of sesquiterpene synthase cDNA by 5' / 3'-RACE
[0096] To isolate the full-length sequence of the sesquiterpene synthase, first use the 5′ / 3′-RACE method with Marathon TM cDNA Amplification Kit (Clontech), starting from 1 μg of mRNA purified from total RNA prepared by Hot Borate technology. The quality of the synthetic cDNA is poor, basically all are small cDNA (average size 0.5Kb). However, with this cDNA, the 3' ends of GFTpsB and GFTpsC were obtained by gene-specific primers, GFTpsBRF1 and GFTpsBRF2 were used for GFTpsB, and GFTpsCRF1 and GFTpsCRF2 were used for GFTpsC (see Table 1). The mRNA prepared by the guanidinium thiocyanate / phenol extraction method obtained higher quality cDNA with an average size of 2Kb, so that the 5'-end sequence of GFTpsC could be isolated with gene-specific primers GFTpsCRR1 and GFTpsRR2 (see Table 1), thereby Complete the full-length sequence of this clone. The 5'-terminal of GFTpsB and the 5'-terminal and 3'-ter...
Embodiment 3
[0103] Example 3: cDNA library screening and EST sequencing
[0104] In order to obtain the partial cloned full-length cDNA obtained by PCR method, a cDNA library prepared from mRNA of pomelo peel was constructed.
[0105] Using Uni-ZAP(R) XR Library Construction Kit (Stratagene), cDNA synthesis and library construction were performed starting from 7.5 μg of grapefruit peel mRNA (prepared by guanidine thiocyanate / phenol method) according to the manufacturer's recommended method. The initial titer of the library was 2 × 10 7 PFU (plaque forming unit), the average length of the insert is 1.1Kb.
[0106] The sesquiterpene synthase encoding cDNA was isolated from the cDNA library described above using two methods: EST sequencing and screening with DNA probes. For EST (Expressed Sequence Tag) sequencing, a portion of the library was used to excise the pBluescript phagemid in the Uni-ZAP XR vector using Stratagene's massexcision protocol. The resulting transformed colonies (576) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com