Fusion protein of single antibody-interleukin 2, its preparation and use
A technology of interleukin and fusion protein, which is applied to the fusion protein of tumor necrosis treatment monoclonal antibody and human interleukin 2, the fusion protein is prepared by genetic engineering, and the application field in diseases can solve the problems that affect the treatment effect, adverse reactions, lack of specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
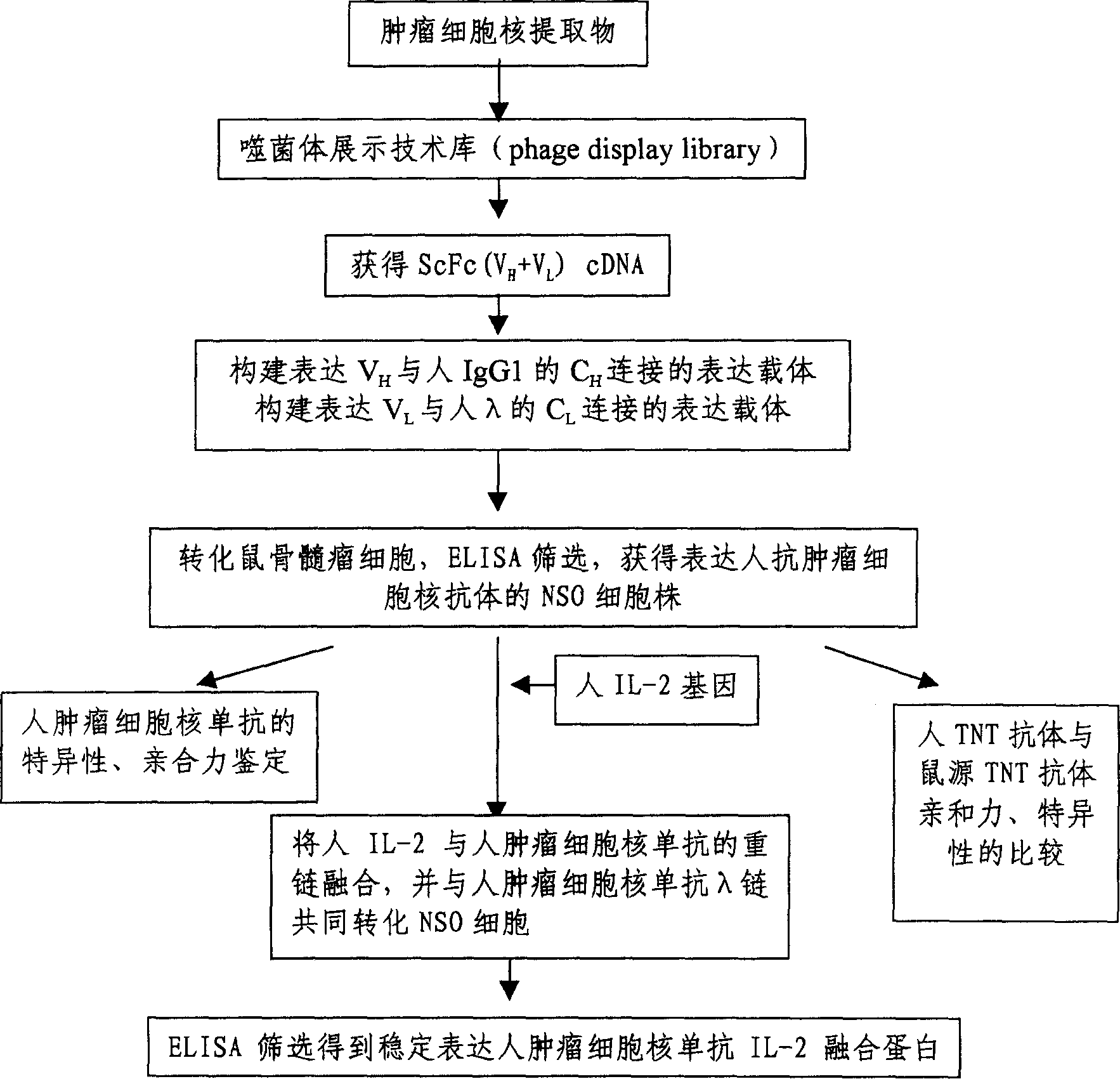
[0076] Construction of high expression cell line expressing rhTNT-IL2 fusion protein
[0077] (a) Preparation of mouse hybridoma cell line NSO-TNT-1 cells
[0078] Immune mice with tumor cell nucleus extracts, then extract and prepare mouse B cell single cell suspensions, fuse with mouse myeloma cells NS-1 through cell fusion and selective culture techniques, continue to repeatedly culture and screen, and use cell immunization The hybridoma cell line NSO-TNT-1, which highly expresses the mouse TNT monoclonal antibody, was screened and stored at low temperature.
[0079] In the following examples, the chimeric tumor necrosis therapeutic monoclonal antibody TNT-1 expressed by the NSO-TNT-1 cell line was used as the experimental positive control of the antibody component of the fusion protein rhTNT-IL2.
[0080] (b) Construction of mouse myeloma cell line NSO-TNT-IL2
[0081] see figure 1 .
[0082] The whole construction process is divided into two steps. Firstly, a positive...
Embodiment 2
[0093] Expression and purification of rhTNT-IL2 fusion protein
[0094] (a) expression
[0095] Take the frozen mouse myeloma cell line NSO-TNT-IL2 (about 1×10 7 cells), recover at 37°C for 2-3 minutes, transfer the cells in the cryopreservation tube to a 15ml centrifuge tube containing 10ml of selective medium, centrifuge the suspended cells at 1500rpm for 10 minutes, discard the supernatant, and place Centrifuge the pelleted cells and inoculate them in the selection medium to start cell expansion culture until the 3L rotary culture flask, the number of cells>2.0×10 6 cells / ml.
[0096] Large-scale culture expression method:
[0097] 30L of culture medium was filtered through a filter device with a pore size of 0.2 μm, and directly filtered into a sterile bioreactor, and the temperature was maintained at 37±0.5°C. All rhTNT-IL2 cells in 3L spinner flasks were transferred to the bioreactor using a peristaltic pump. Inoculation was performed 36 hours after the medium was f...
Embodiment 3
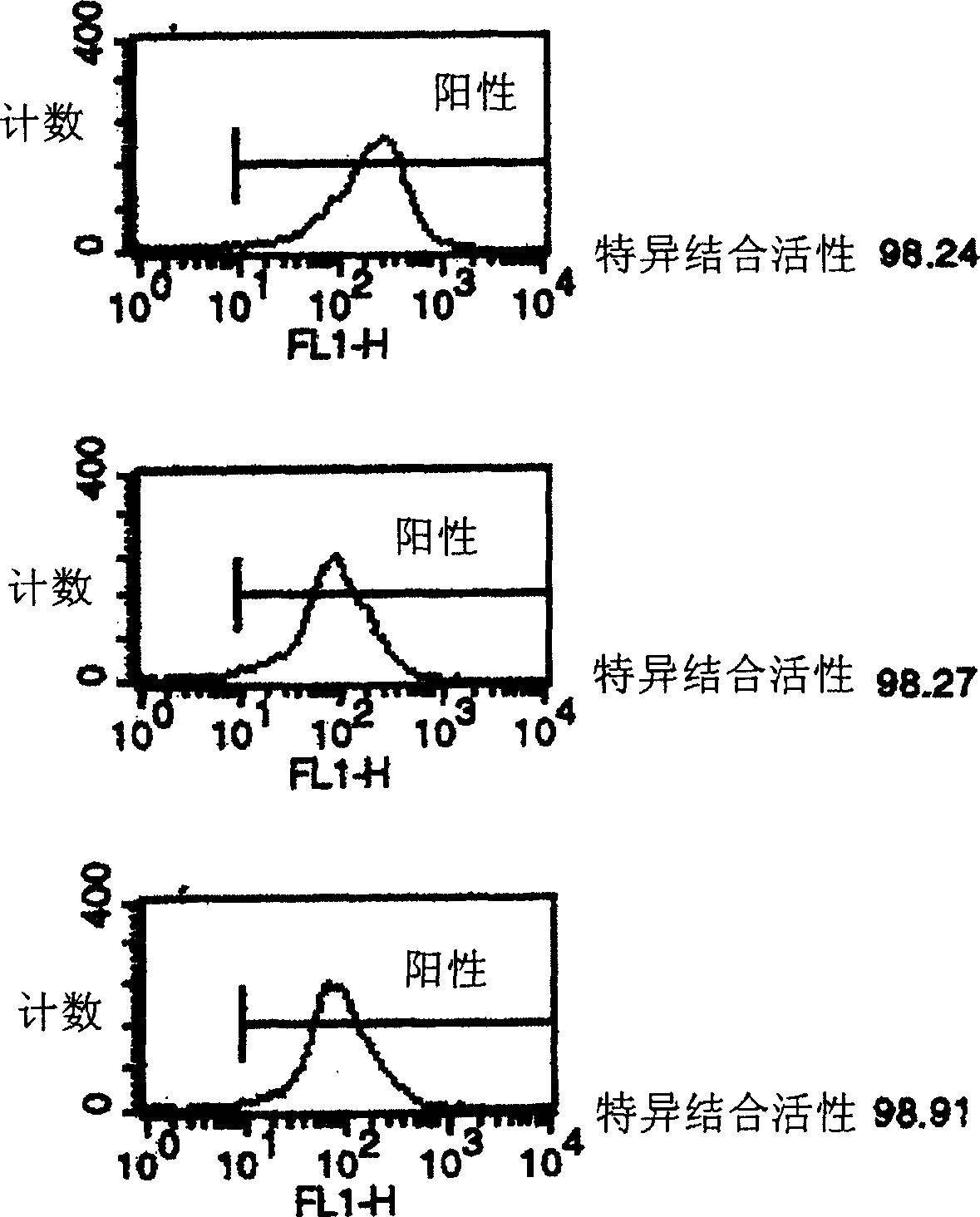
[0113] Detection of rhTNT-IL2 fusion protein
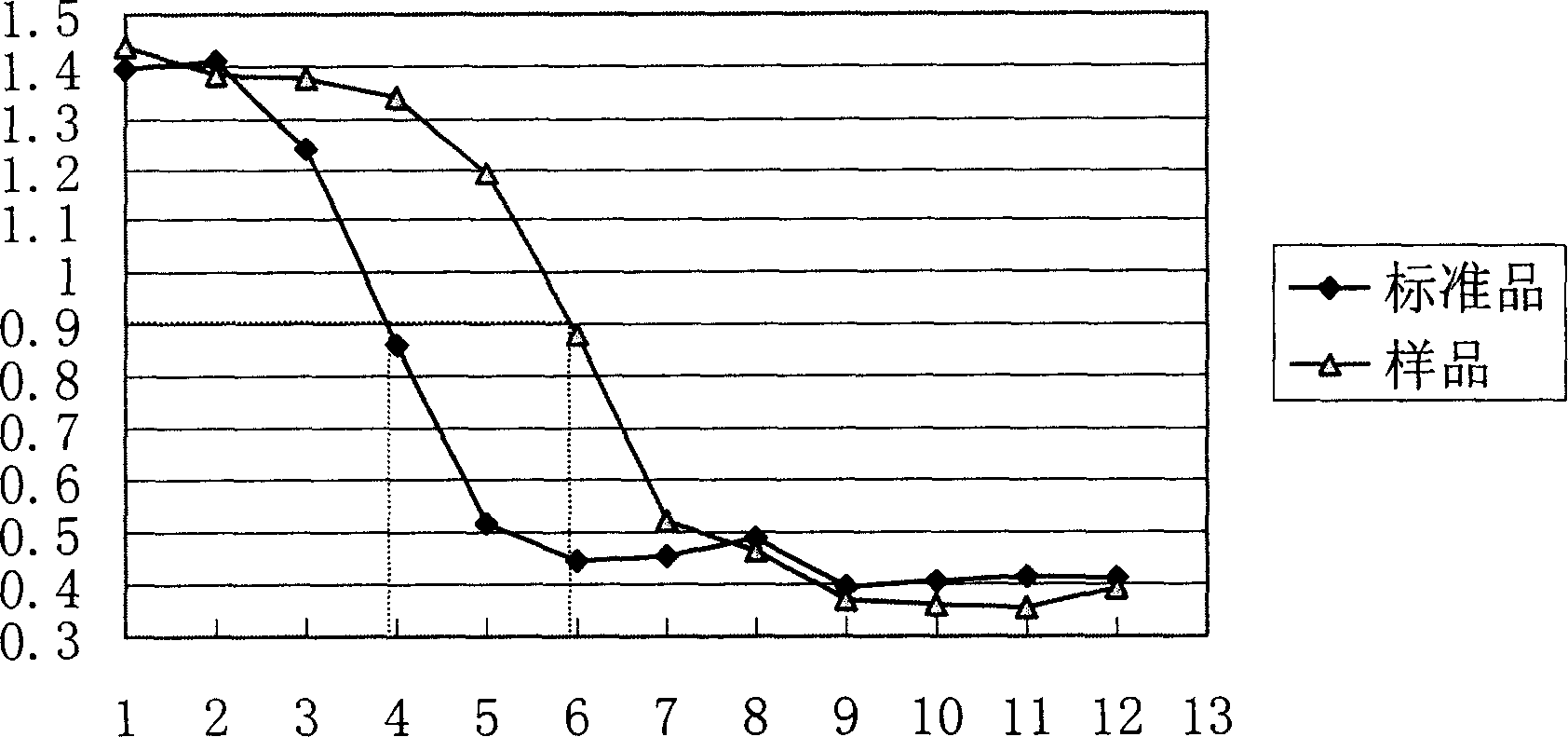
[0114] (a) IL2 titer determination
[0115] Performed with conventional CTLL-2-dependent cell line / MTT colorimetry. Methods as below:
[0116] (1) Preparation of cell suspension: take a sufficient amount of CTLL-2 cells (ATCC TIB-214) to collect by centrifugation, wash 3 times with basal solution (RPMI1640+10% calf serum), and then resuspend in basal culture medium, Formulated to 5×10 5 / ml of cell suspension and stored at 37°C.
[0117] (2) Preparation of standard solution: Take 1 standard product (2000IU) and dissolve it according to the instruction manual, then dilute to 200IU / ml with basic culture solution.
[0118] (3) The sample to be tested (stock solution, according to 1×10 6 IU / mg calculation) was diluted to 200IU / ml with basal culture medium.
[0119] (4) In the 96-well cell culture plate, the prepared standard solution (200IU / ml) and the sample (200IU / ml) solution are continuously diluted in a doubling ratio to ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com