Genetically engineered hematopoietic stem cell drug delivery system as well as preparation method and application thereof
A hematopoietic stem cell and genetic engineering technology, applied in the field of genetically engineered hematopoietic stem cell drug delivery system and its preparation, can solve the problems of loss of cell biological activity, CFDV can't specifically target organs, and can't respond to chemical signals, etc., to achieve Reduce immunotoxicity, low immunogenicity, simple purification and culture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Construction of a genetically engineered hematopoietic stem cell drug delivery platform (SB@HSCs-PD-1)
[0047] Configuration of TGF-β inhibitor solution: 10 mg / mL solution was prepared in DMSO, and then diluted in PBS to configure different concentration gradients.
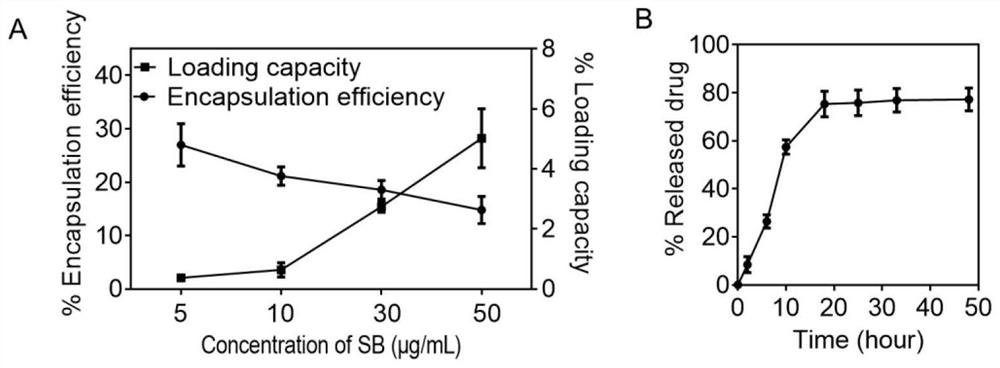
[0048] After incubating the TGF-β inhibitor with a concentration gradient with hematopoietic stem cells for 4 hours, centrifugation at 500 g for 3 minutes, collecting the supernatant, and detecting its loading and drug release by high performance liquid chromatography. The result is as figure 1 As shown, after HSCs were incubated with TGF-β inhibitor at a concentration of 50 μg / mL, the encapsulation efficiency was 14.78 ± 2.55%, and the loading rate was 5.02 ± 0.98 wt%. 7.39±0.49 μg TGF-β inhibition. TGF-[beta] inhibition in phosphate buffer at 37[deg.]C released about 77.24±4.75% in 48 hours.
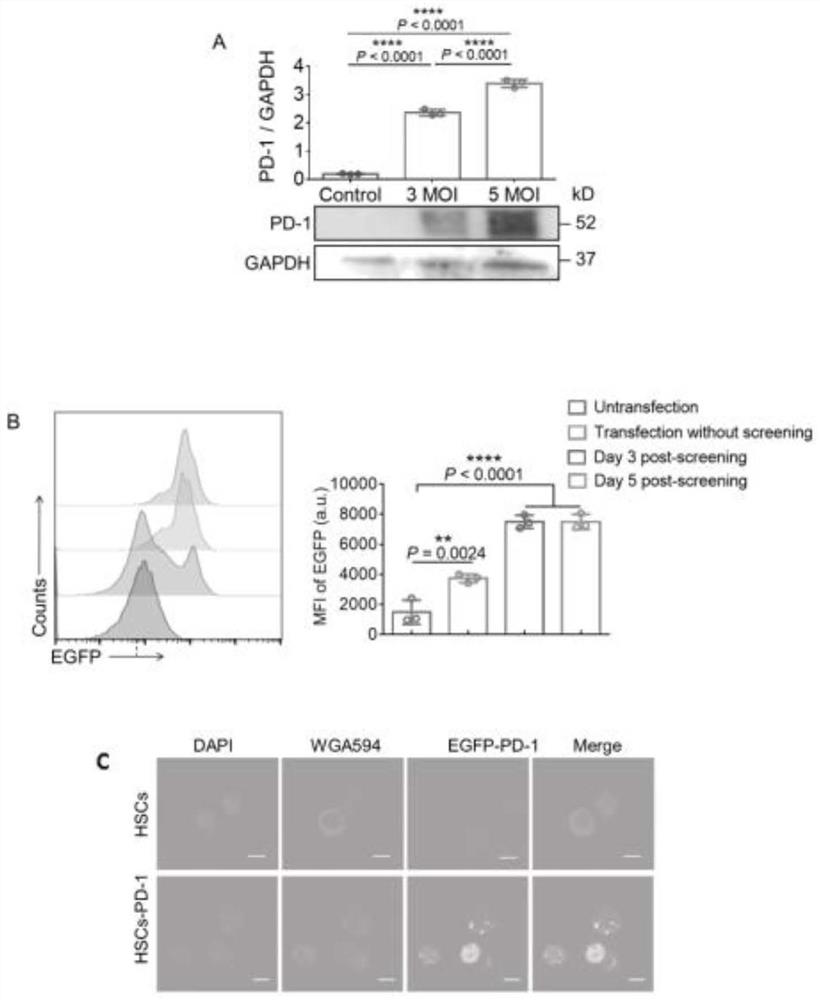
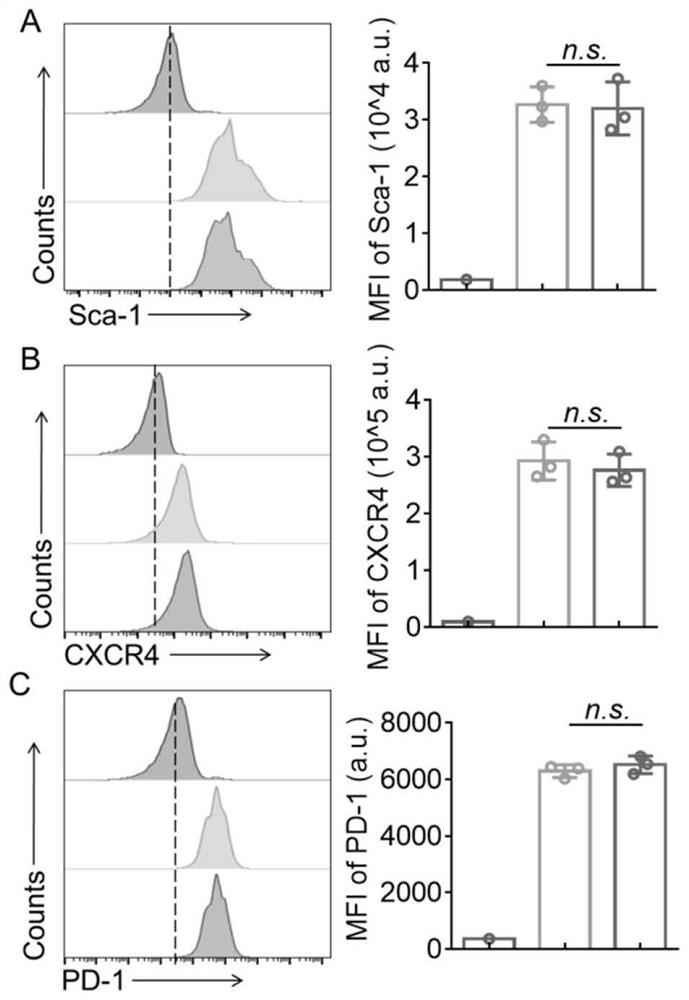
[0049] The purified HSCs were co-incubated with the lentiviral vector (pRLenti-EF1-EGFP-P2A-Puro-CM...
Embodiment 2
[0051] Example 2 Correlation characterization of SB@HSCs-PD-1
[0052] (1) Binding ability of HSCs-PD-1 to PD-L1 antibody
[0053] The melanomas with high PD-L1 expression were prepared by transfecting lentivirus (HBLV-m-CD274-3xflag-ZsGreen-PURO) to detect the PD-L1 binding ability of HSCs-PD-1. The results are as follows Figure 4 As shown, the transformed melanoma cells not only stably express ZsGreen signal, but also highly express PD-L1. Suspended HSCs-PD-1 and HSCs were separately co-cultured with adherent PD-L1-expressing melanoma cells B16F10 for 3 hours, and unbound supernatant cells were removed by washing with PBS. The remaining cells were stained with DAPI and PE-CD44 antibodies and the results were as follows Figure 5 shown, HSCs-PD-1 co-incubation showed a significant increase in CD44 signal compared to cancer cells co-cultured with HSCs, suggesting that HSCs-PD-1 can adhere to B16F10-PD-L1 via PD-1 / PD-L1 binding on cells.
[0054] (2) Bone homing ability of...
Embodiment 3
[0058] Example 3 Therapeutic effect of SB@HSCs-PD-1 on bone metastases
[0059] The mice were administered with PBS (200 μL) and HSCs (1 × 10 via tail vein) on the third day after the establishment of the bone metastases model. 5 cells), SB (7.5 μg), HSC-PD-1 (1×10 5 cells), SB@HSCs (1×10 5 cells) and SB@HSCs-PD-1 (1×10 5 cells), injected every three days for a total of 4 treatments. The size of bone metastases was measured by small animal imaging at specific time points and the survival of mice in each group was recorded.
[0060] The result is as Figure 8 As shown, in mice receiving PBS, HSC, SB alone, the bioluminescence signal of bone metastases increased rapidly, indicating that bone metastases do not respond to antibodies to PD-L1. Bone metastatic burden was slightly reduced in mice treated with SB@HSCs or HSCs-PD-1 compared with control mice, and after treatment with the genetically engineered hematopoietic cell delivery system (SB@HSCs-PD-1), bone metastases were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com