Endomorphin analog and its preparing method
An endomorphin and analog technology, applied in the field of new compounds and their preparation methods and pharmacological efficacy, can solve the problems of limited application of analgesics, dose dependence, limited application, etc. Pain-specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
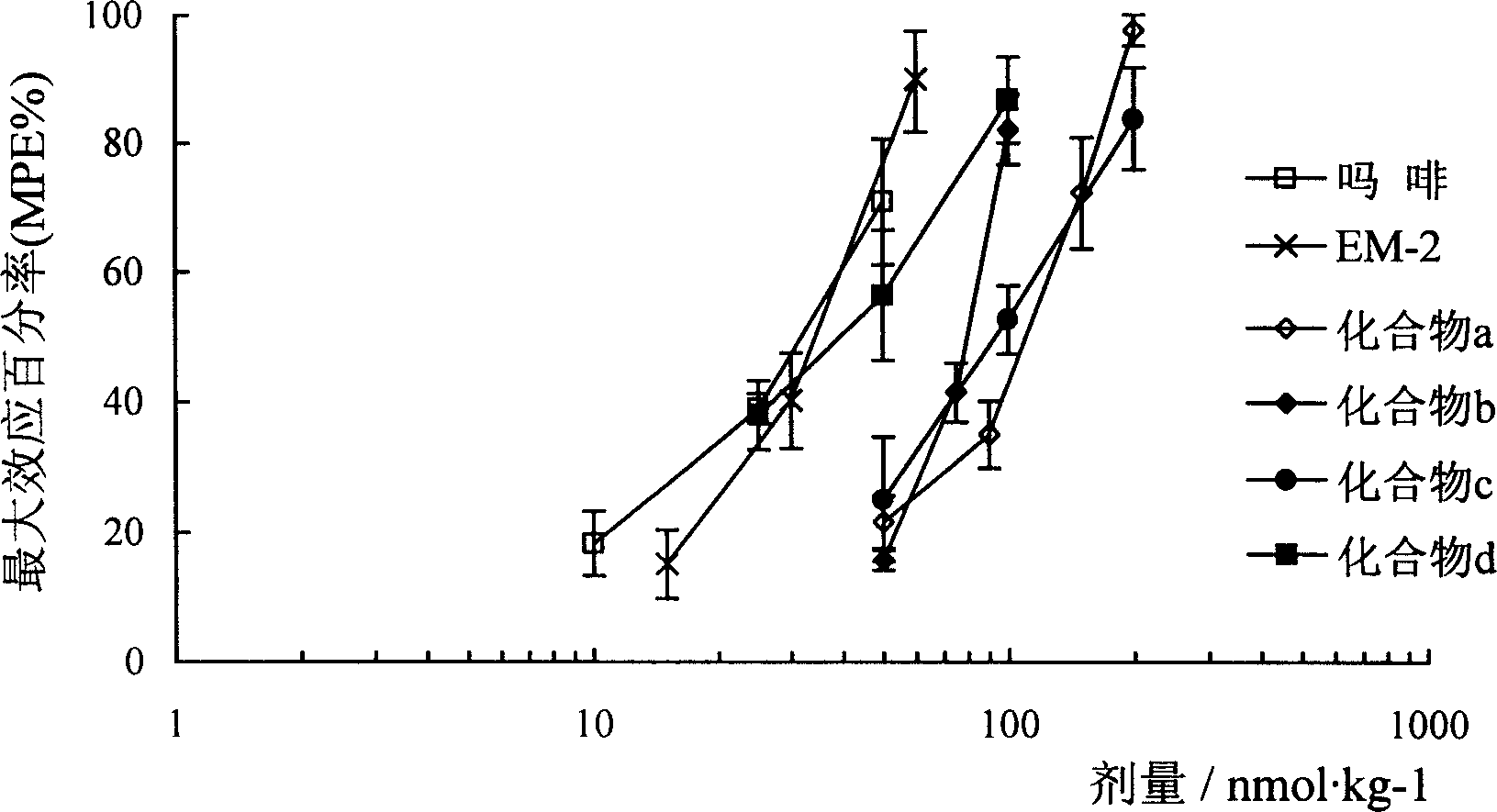
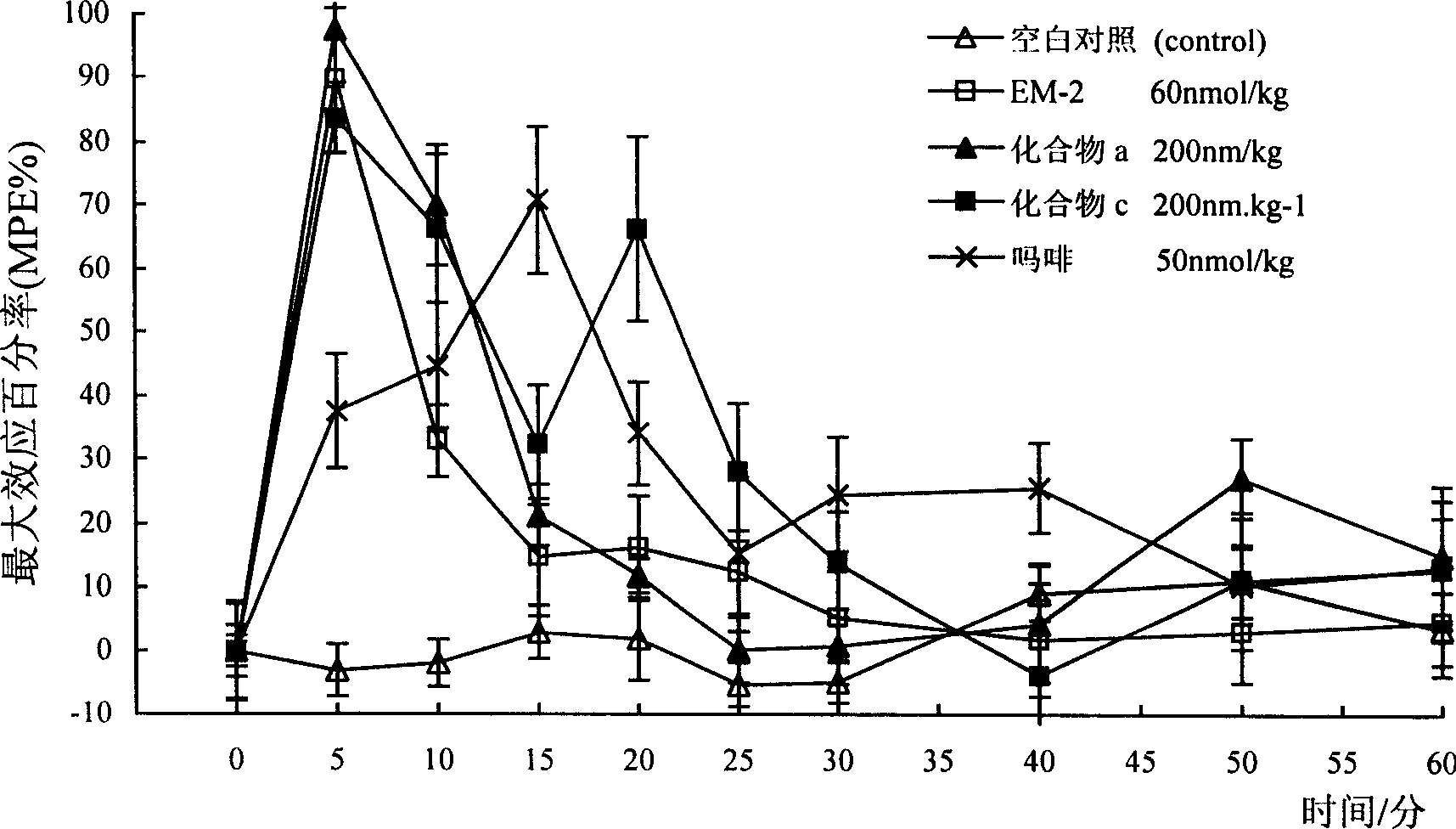
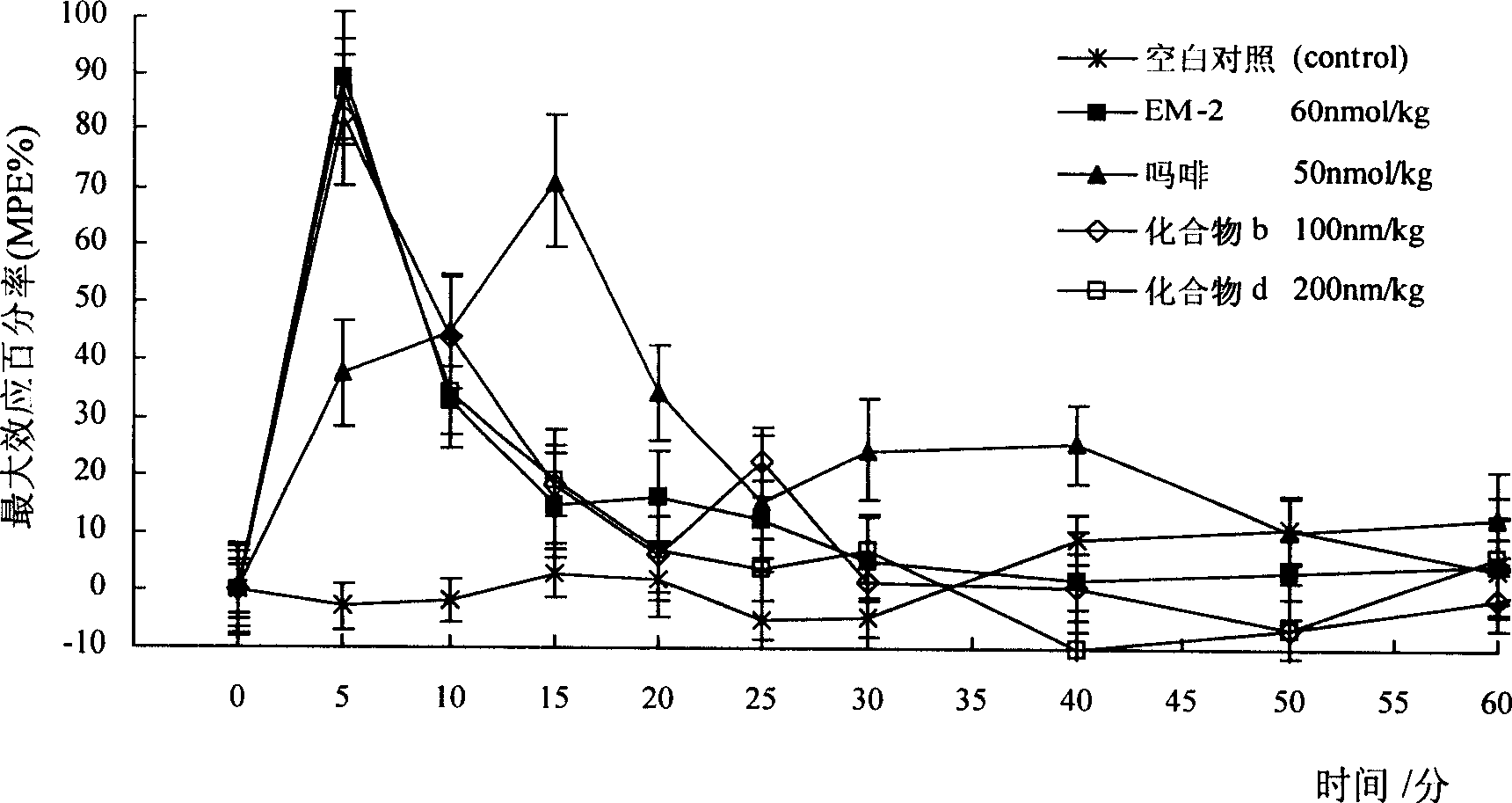
Image
Examples
Embodiment 1
[0040] Embodiment 1: compound c (Tyr-D-Pro-Phe-Gly-NHCH (CH 3 ) Ph, the synthesis of tyrosyl-D type-prolylphenylalanylglycyl-L-α-methylbenzylamine)
[0041] Compound i (Z-Gly, N-benzyloxycarbonylglycine) was dissolved in anhydrous DCM (adding anhydrous DMF to aid dissolution), ice bathed, and 1.5 times the amount of L-(-)α-methylbenzylamine was added, and then Ice-bathed for 10 minutes, then added 1.5 times the amount of DCC dissolved in pre-cooled dichloromethane (DCM), stirred in the ice bath for 30 minutes, added 0.5 times the amount of DMAP, after 30 minutes, returned to room temperature and reacted for 40 hours, filtered off DCU, The filtrate was concentrated under reduced pressure to remove the solvent, dissolved in ethyl acetate, and then washed with dilute hydrochloric acid, saturated NaHCO 3 , NaCl solution, and dried over anhydrous sodium sulfate. The crude product was recrystallized with EA / PE (1:2, V / V) to obtain compound ii (Z-Gly-NHCH(CH 3 )Ph): yield = 86.7%;...
Embodiment 2
[0048] Embodiment 2: compound a (Tyr-Pro-Phe-Gly-NHCH (CH 3 ) Ph, the synthesis of tyrosylprolylphenylalanylglycyl-L-α-methylbenzylamine)
[0049] Compound viii (Phe-Gly-NHCH(CH 3 ) Ph) structure and synthesis process are the same as in Example 1.
[0050] Dissolve equimolar amounts of Z-Tyr and HOSu in anhydrous THF, slowly drop into the pre-cooled THF solution in which an equivalent amount of DCC was dissolved, and react overnight at room temperature under cooling at 3 After the solution was dissolved, it was added dropwise into the Z-Tyr-OSu solution, and reacted at room temperature for about 2 days. After the reaction was finished, 6 mol / L HCl was slowly added dropwise until the pH of the solution was 2. It was then concentrated under reduced pressure to remove THF, extracted with ethyl acetate, washed with acid, then washed with NaCl solution until neutral, and dried by adding anhydrous sodium sulfate. Anhydrous sodium sulfate was filtered off, and concentrated under r...
Embodiment 3
[0053] Embodiment 3: compound d (Tyr-D-Pro-Phe-Ala-NHCH (CH 3 ) Ph, the synthesis of tyrosyl-D type-prolylphenylalanylalanyl-L-α-methylbenzylamine)
[0054] See Example 1 for the synthesis of compound vii (Z-Tyr-D-Pro) in Example 3; compound xii (Phe-Ala-NHCH(CH 3 )Ph) synthetic process and concrete operation and embodiment 1 compound viii (Phe-Gly-NHCH(CH 3 ) Ph) is similar, except that the raw material compound i (Z-Gly) in Example 1 is changed to compound xiii (Z-Ala, N-benzyloxycarbonylalanine) to react to obtain xii (Phe-Ala-NHCH( CH 3 )Ph).
[0055] Dissolve equimolar amounts of compound vii (Z-Tyr-D-Pro) and HOBt in anhydrous DCM / DMF mixed solvent, and slowly drop into the pre-cooled DCM solution in which equimolar amounts of DCC were dissolved under ice-salt bath cooling and stirring , After reacting at room temperature for 12h, filter off DCU to obtain Z-Tyr-D-Pro-OBt solution. Ice-salt bath, stirring, adding 1.2 times the amount of compound xii (Phe-Ala-NHCH(CH ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com