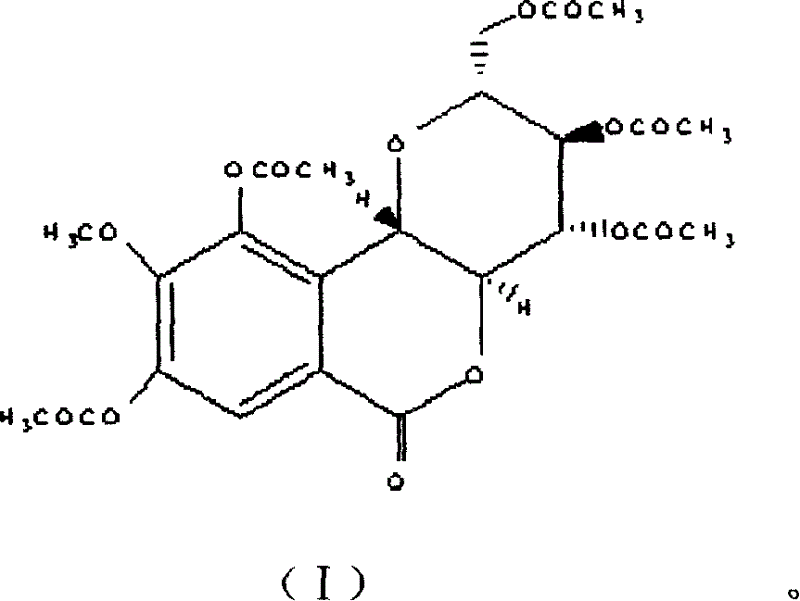
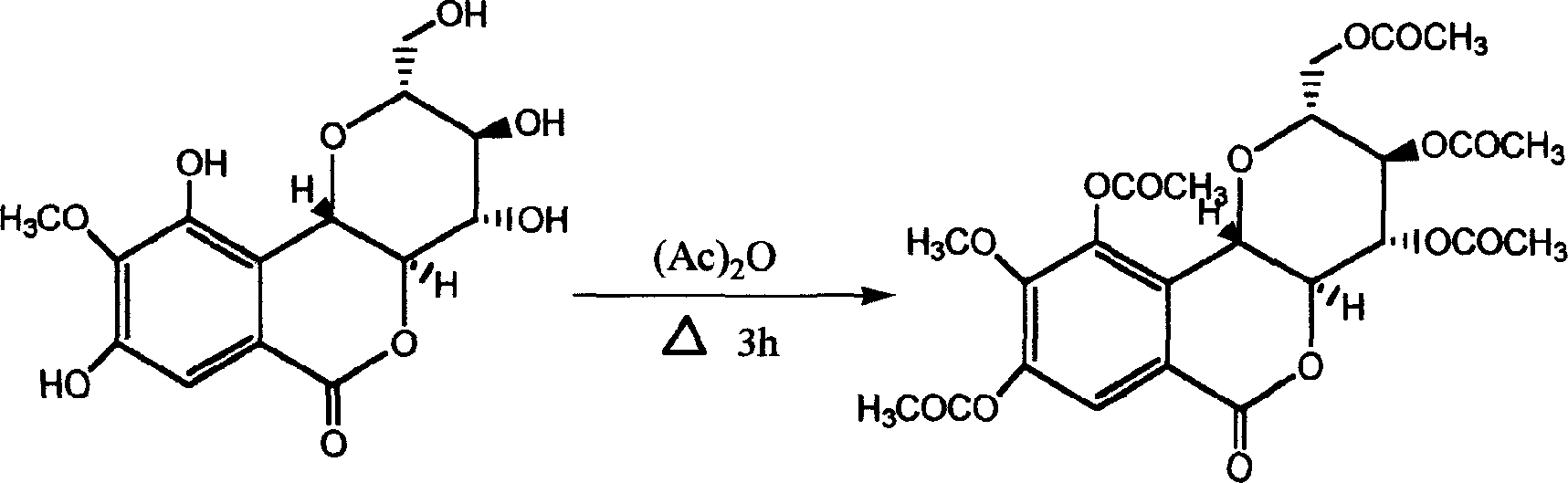
Purple bergenia element pentaacetylate and its uses
A technology of pentaacetylated compound and petogenin, which is applied in the field of medicine, can solve the problems of poor oral absorption, weak antitussive and antiasthmatic effects, etc., to overcome poor oral absorption, weak antitussive and antiasthmatic effects, and obvious drug potentiating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] Example 1: Weigh 5g of petracenin and place it in a 250ml reaction bottle, add 18g of sodium acetate and 100ml of acetic anhydride, heat and stir at 60°C, and react for 3 hours, pour the reaction solution into 500ml of distilled water, and place in a refrigerator at 4°C overnight , a white solid precipitated, the precipitate was washed with water until neutral, and recrystallized with chloroform-petroleum ether with a volume ratio of 5:1 to obtain about 5 g of white granular crystals, with a yield of about 80%, mp: 203-205 ° C, This product is easily soluble in chloroform, acetone, and ether, but less soluble in methanol and ethanol.
[0009] EI-MS: 538(5)[M + ], 496(100), 454(10), 412(15), 376(65), 292(90), 274(65).
[0010] 1 H-NMR (CDCl 3 , 400MHz): 7.06(1H, s, H-4), 4.93(1H, d, J=10.4, H-1'), 4.15(1H, m, H-2'), 4.10(1H, m, H -6a'), 3.88 (3H, s, OCH 3 ), 3.85(1H, t, J=9.0, H-3'), 3.75(1H, m, H-6b'), 3.65(1H, m, H-5'), 3.52(1H, t, J= 9.0, H-4'), 2.10-1.95 (15H, ...
Embodiment 2
[0012] Example 2: Weigh 10g of petracenin and place it in a 500ml reaction bottle, add 30g of sodium acetate and 250ml of acetic anhydride, heat and stir at 90°C, and react for 5 hours, pour the reaction solution into 1000ml of distilled water, and place in a refrigerator at 4°C overnight , a white solid was precipitated, the precipitate was washed with water until neutral, and recrystallized with chloroform-petroleum ether with a volume ratio of 5:1 to obtain about 9 g of white granular crystals, with a yield of about 75%.
Embodiment 3
[0013] Example 3: Weigh 1 g of petracenin and place it in a 50 ml reaction bottle, add 3 g of sodium acetate, 50 ml of acetic anhydride, heat and stir at 55°C, and react for 1.5 hours, pour the reaction solution into 100 ml of distilled water, and place in a refrigerator at 4°C overnight , a white solid was precipitated, the precipitate was washed with water until neutral, and recrystallized with chloroform-petroleum ether with a volume ratio of 5:1 to obtain about 0.8 g of white granular crystals, with a yield of about 80%.
[0014] The role of petracenin pentaacetyl in antitussive and antiasthmatic is confirmed by animal experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com