Selective androgen receptor modulators and methods of use thereof
An androgen receptor, selective technology, applied in the direction of diseases, pharmaceutical formulations, organic active ingredients, etc., can solve the problem of non-steroidal androgen reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Non-steroidal ligands with androgenic and anabolic activity
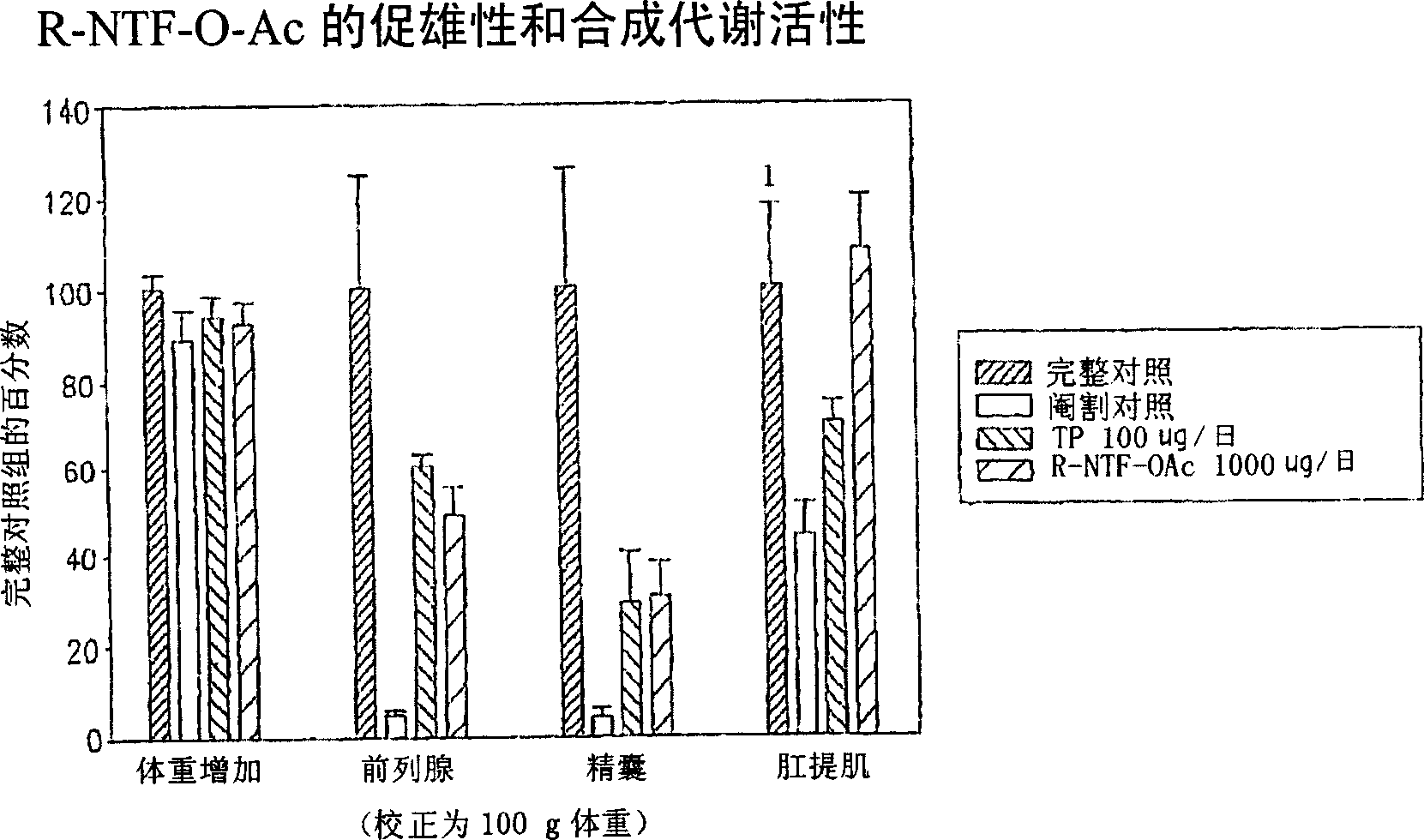
[0189] The SARM compounds provided herein were designed, synthesized, and evaluated for their in vitro and in vivo pharmacological activities. In vitro androgen receptor binding affinity and ability to maintain androgen-dependent tissue growth in castrated animals were studied. Androgenic activity is monitored by the ability of the SARM compounds to maintain and / or stimulate prostate and seminal vesicle growth, which is measured gravimetrically. Anabolic activity is monitored by the ability of the SARM compounds to maintain and / or stimulate the growth of the levator ani muscle measured gravimetrically.
[0190] The synthetic process of compound I-VIII:
[0191] (2R)-1-Methacryloylpyrrolidine-2-carboxylic acid (R-129). D-Proline (R-128, 14.93 g, 0.13 mol) was dissolved in 71 mL of 2N NaOH and cooled in an ice bath; the resulting basic solution was diluted with acetone (71 mL). A solution of meth...
Embodiment 2
[0200] Non-steroidal ligands with androgenic and anabolic activity
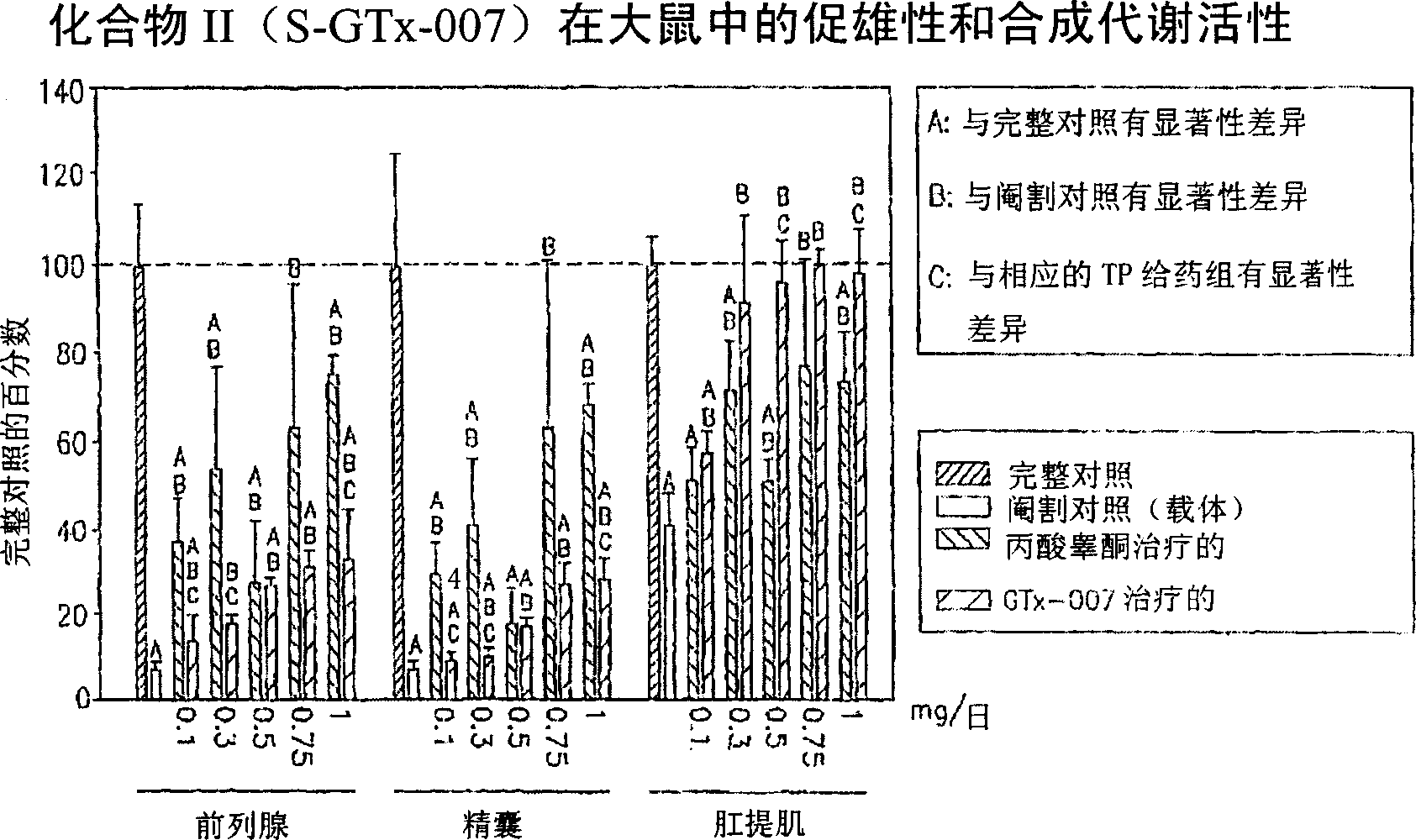
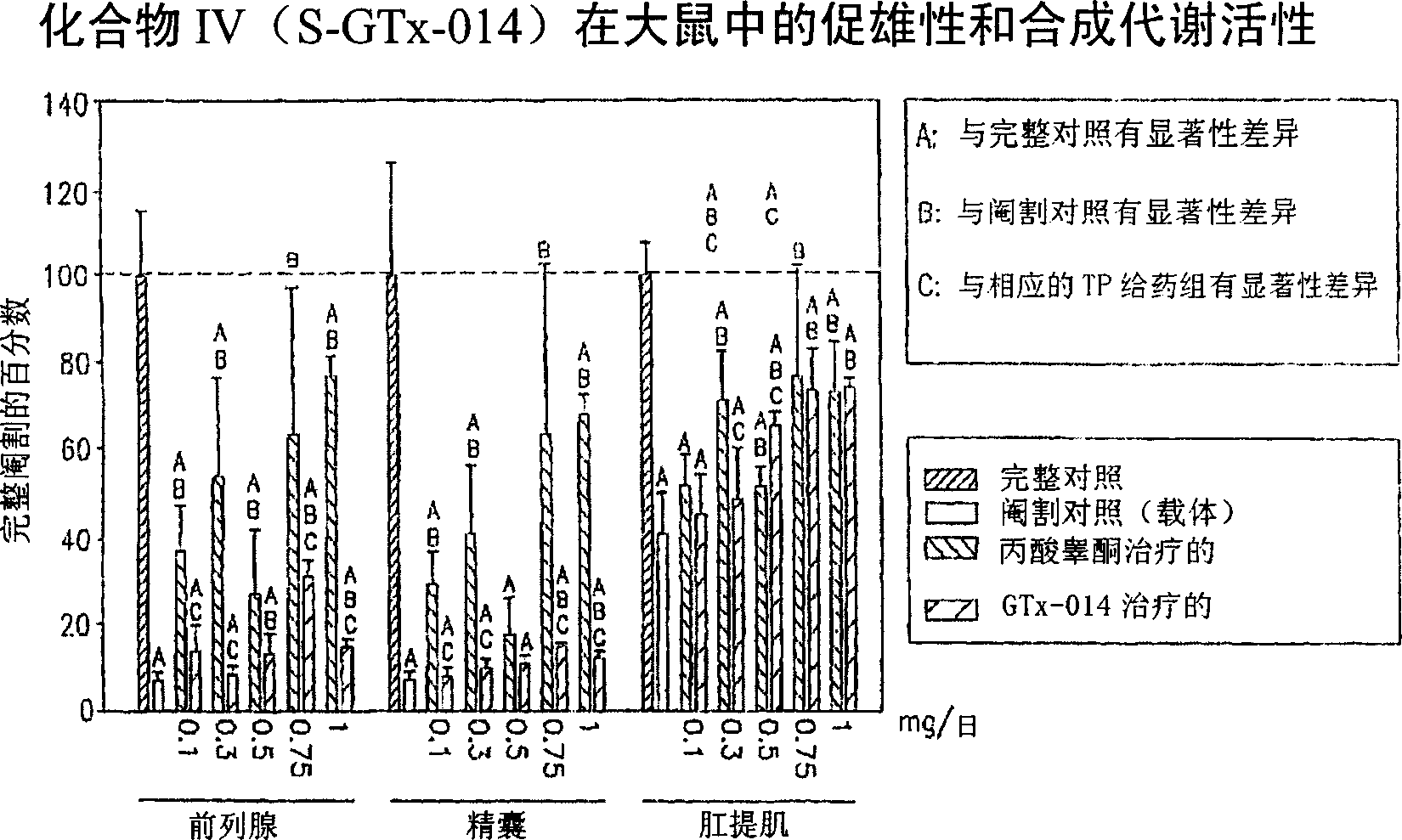
[0201] The in vivo efficacy and acute toxicity of four new non-steroidal androgens (compounds IV, V, VI and VII) were examined in rats. In vitro experiments confirmed that these compounds bind the androgen receptor with high affinity. The structures and names of the four compounds are as follows:
[0202]
[0203] GTx-014 R=F
[0204] GTx-015 R=COCH 3
[0205] GTx-016 R=COC 2 h 5
[0206] GTx-007 R=NHCOCH 3
[0207] experiment method
[0208] material.according to Figure 9 The shown route synthesizes compound GTx-014 (compound IV), GTx-015 (compound V), GTx-016 (compound VI) and GTx-007 (compound VII, wherein R is NHCOCH 3 ) and the R-isomer of GTx-014. Testosterone propionate (TP), polyethylene glycol 300 (PEG300, reagent grade) and neutral buffered formalin (10% w / v) were purchased from Sigma Chemical Company (St Louis, MO). Alzet ...
Embodiment 3
[0225] Pharmacokinetics of GTx-007 in dogs
[0226] The pharmacokinetics of S-GTx-007, a novel selective androgen receptor modulator (SARM), were characterized in beagle dogs. A four-treatment, four-period crossover design was used in the study involving a total of six beagles, three of each sex. Each animal received a 3 mg / kg IV dose, a 10 mg / kg IV dose, a 10 mg / kg PO dose in solution and a 10 mg / kg PO dose in capsules in the order of random allocation. There is a one-week washout period between treatments. Plasma samples were collected up to 72 hours after drug administration. Plasma S-GTx-007 concentrations were analyzed by a certified HPLC method. Determination of clearance (CL), volume of distribution (Vss), half-life (T 1 / 2 ) and other pharmacokinetic parameters. The results showed that after IV administration, S-GTx-007 cleared terminal T from canine plasma. 1 / 2 About 4 hours, CL is 4.4mL / min / kg. Figure 4 , 5 and 6 show the plasma concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com