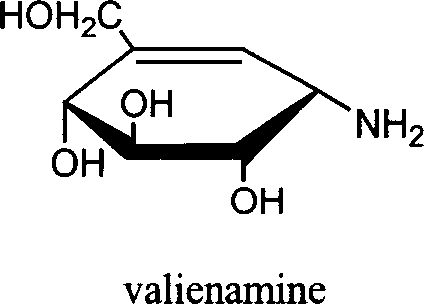
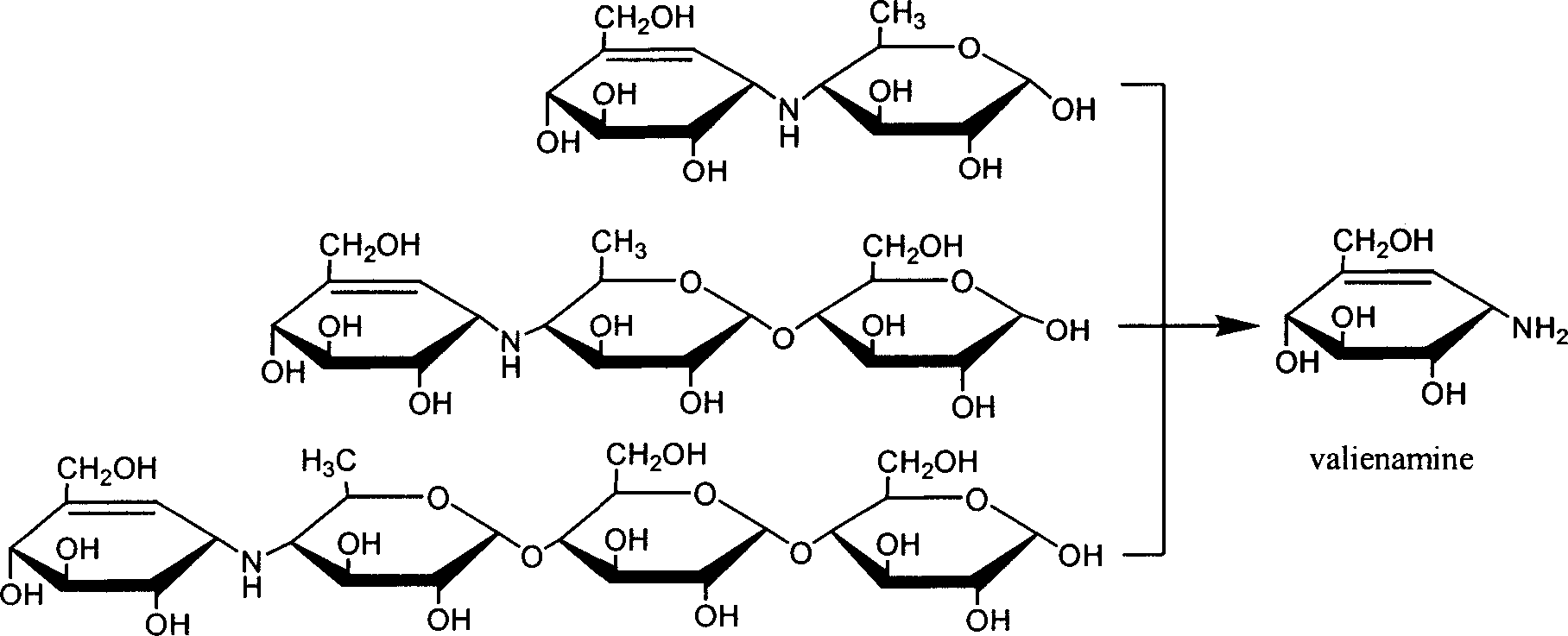
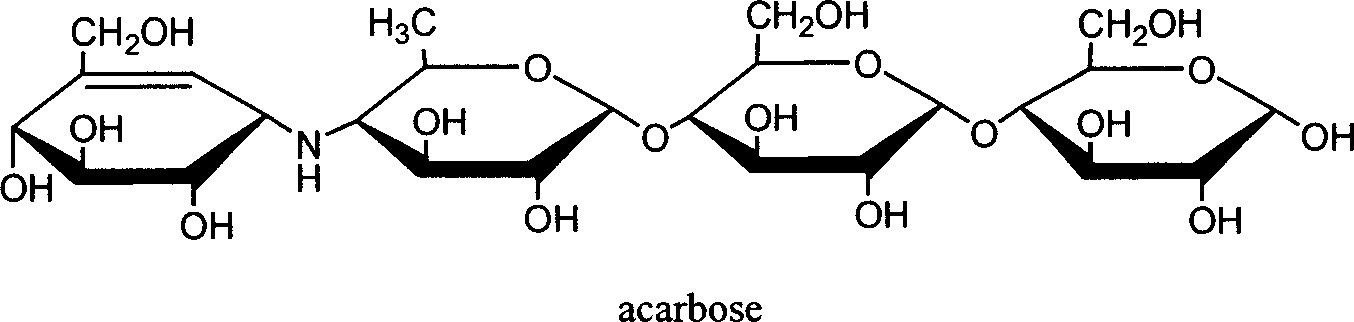
Preparation of effective mycoenamine with microbial lytic acrose and its derivative
A technology for acarbose and derivatives, which is applied in the field of preparing effective memtenamine, can solve the problems of no industrialized production, low yield, complex lysate components, etc., and achieves low production cost, huge economic benefits, and good application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Fermentation medium formula (weight / volume percentage, the same below): Acarbose 2.0%, (NH 4 ) 2 SO 4 1.5%, KCl 0.5%, Na 2 HPO 4 12H 2 O 1.0%, MgSO 4 0.02%, prepared with tap water, adjusted to pH 6.0, and set aside.
[0038] Take 150mL of the above-mentioned culture medium, evenly distribute it in three 250mL Erlenmeyer flasks, and sterilize. Inoculate the slant strain CCTCC No.M 205091, cultivate the bacteria, shake the rotating speed of 200r / min, and cultivate it at 30°C for 48 hours as the seed liquid, and set aside.
[0039] Take 2L of the above culture medium, divide it into 20 500mL shake flasks, and sterilize. The seed solution was inserted, the inoculum amount was 5% (v / v), and culture was carried out at a culture temperature of 28° C., a shaker speed of 200 r / min, and a fermentation time of 120 h.
[0040] Collect 1.8L of the above-mentioned fermentation broth, centrifuge to remove the bacteria, and put the supernatant on the column (D113 resin, NH 4...
Embodiment 2
[0042] Fermentation medium formula: acarbose 1.0%, (NH 4 ) 2 SO 4 0.5%, KCl 0.1%, Na 2 HPO 4 12H 2 O 0.1%, MgSO 4 0.02%, prepared with tap water, adjusted to pH 7.0, and set aside.
[0043] Take 150mL of the above-mentioned seed culture medium, evenly distribute it in three 250mL Erlenmeyer flasks, and sterilize. Insert the slant strain CCTCC No.M205091, cultivate the bacteria, shake the rotating speed of 150r / min, and cultivate it at 28°C for 48 hours as the seed solution, and set aside.
[0044] Take 2L of the above culture medium, divide it into 20 500mL shake flasks, and sterilize. The seed solution was inserted, the inoculum amount was 5% (v / v), and culture was carried out, the culture temperature was 30° C., the rotation speed of the shaker was 200 r / min, and the fermentation time was 72 hours.
[0045] 1.8 L of the above-mentioned fermentation broth was collected, separated and purified, and the steps were the same as in Example 1 to obtain 1.1 g of effective ...
Embodiment 3
[0047] Fermentation medium formula: acarbose 2.5%, (NH 4 ) 2 SO 4 2.0%, KCl 1.0%, Na 2 HPO 4 12H 2 O 2.0%, MgSO 4 0.1%, prepared with tap water, adjusted to pH 8.0, and set aside.
[0048] Take 150mL of the above-mentioned culture medium, evenly distribute it in three 250mL Erlenmeyer flasks, and sterilize. Inoculate the slant strain CCTCC No.M 205091, cultivate the bacteria, shake the rotating speed of 200r / min, and cultivate in the shaking table at 32°C for 48h as the seed liquid, and set aside.
[0049] Take 2L of the above culture medium, divide it into 20 500mL shake flasks, and sterilize. The seed solution was inserted, the inoculum amount was 5% (v / v), and culture was carried out, the culture temperature was 32° C., and the shaker speed was 200 r / min. The fermentation time is 144h.
[0050] 1.8 L of the above-mentioned fermentation broth was collected, separated and purified, and the steps were the same as in Example 1 to obtain 3.0 g of effective mycylamine. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com