Polyphenol hydroxy flavone compound with pharmaceutical function and preparation method thereof
A compound and mixture technology, applied in the field of medicine, can solve the problems of difficult extraction and separation, scarce quantity and high cost, and achieve the effects of stable and reliable supply and quality, mature production technology and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
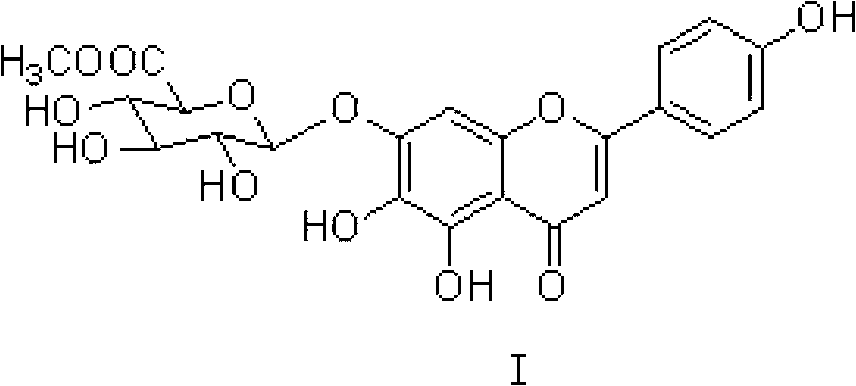
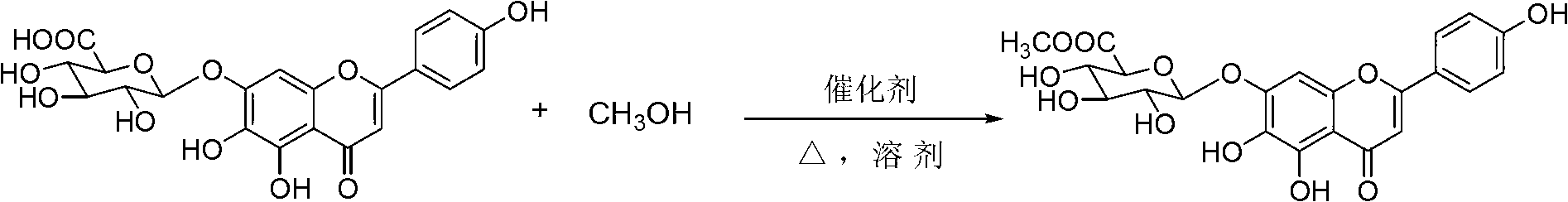
[0045] Example 1 Synthesis of the compound of formula I (the catalyst used is CaCl 2 )
[0046] Take 28.0g of scutellarin raw material (equivalent to scutellarin 23.1g, 0.05mol), put it in a 1000mL round bottom flask, add 500g methanol, 0.56g CaCl 2 (0.005mol), heated and stirred at 50°C for 2h. The reaction solution was concentrated by vacuum to recover methanol. When the volume of the concentrated solution was reduced to about 1 / 3 of the original volume, the concentration was stopped, and the concentrated solution was allowed to stand and cool to room temperature. A large amount of product crystallized out. Vacuum filter, wash with 30mL cold methanol three times, try to drain, transfer the obtained product into 250mL ethyl acetate-petroleum ether (3:1), heat to boiling for 30min, put it in the refrigerator at 0-5℃ to cool for more than 4h . Take out the crystalline product, vacuum filter, wash with 30mL cold ethyl acetate three times, and finally wash with about 15mL petroleum...
Embodiment 2
[0047] Example 2 Synthesis of the compound of formula I (the catalyst used is ZnCl 2 )
[0048] Take 28.0g of scutellarin raw material (equivalent to scutellarin 23.1g, 0.05mol), put it in a 1000mL round bottom flask, add 500g methanol, 0.68g ZnCl 2 (0.005mol), heated and stirred at 50°C for 2h. The rest is the same as in Example 1 to obtain 23.3 g of the compound of formula I in a light yellow loose powder form with a yield of 97.9%.
Embodiment 3
[0049] Example 3 Synthesis of the compound of formula I (the catalyst used is MgCl 2 )
[0050] Take 28.0g of scutellarin raw material (equivalent to scutellarin 23.1g, 0.05mol), put it in a 1000mL round bottom flask, add 500g methanol, 0.48g MgCl 2 (0.005mol), heated and stirred at 50°C for 2h. The rest is the same as in Example 1 to obtain 19.3 g of the compound of formula I in a light yellow loose powder form with a yield of 81.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com