Synthesis method of (R,S)-S-secondary butyl o-ethyl-2-oxo-1,3-thiazolidine-3-thiosulphate
A technology for the synthesis of ethylphosphorothioate and its application in (R,S)-S-sec-butyl-O-ethyl-2-oxo-1,3-thiazolidin-3-ylthio In the field of phosphate ester synthesis, it can solve the problems of low intermediate content, affecting product content, complex synthesis process, etc., and achieve the effects of high yield, simple operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
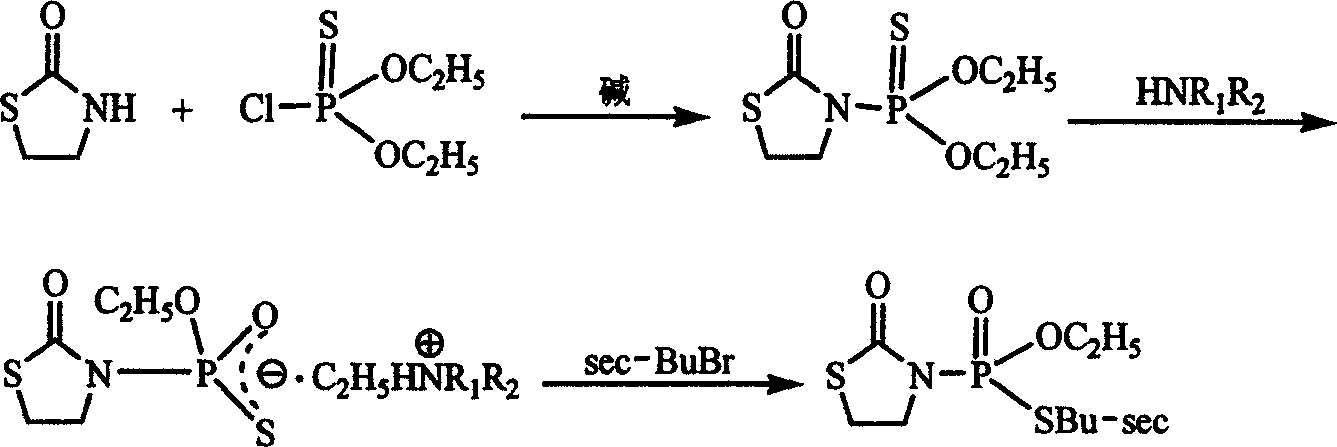
[0022] In a 500 mL four-necked flask, add 31-2 g (0.3 mol) 2-thiazolidinone, 42.2 mL (0.3 mol) triethylamine and 200 mL toluene, and stir with electric power. Heat to 100°C, and add 67.5mL (0.42mol) O,O'-diethylthiophosphoryl chloride dropwise. After the dripping is completed, the heat preservation reaction is continued for 4 hours. After the reaction is complete, cool to room temperature, wash with water, dry the toluene layer with anhydrous sodium sulfate, filter, and desolvate to obtain brown-yellow O,O'-diethyl-2-oxo-1,3-thiazolidine-3-yl Thiophosphate.
[0023] Add the above-mentioned O, O'-diethyl-2-oxo-1,3-thiazolidine-3-ylthiophosphate (in 0.276 mol) and 82.5 mL (0.55 mol) 33 into a 250 mL four-necked flask. % Dimethylamine aqueous solution, stir, heat to reflux, keep the temperature and react for 20 hours. The reaction was stopped, and the reaction solution was concentrated to obtain a pale yellow viscous phosphorothioate ammonium salt. The above-mentioned phosphorothioat...
Embodiment 2
[0029] In a 500 mL four-necked flask, 31.2 g (0.3 mol) of 2-thiazolidinone, 27.2 mL (0.33 mol) of pyridine and 200 mL of toluene were added, and stirred with electric power. Heat to 90°C, and add 57.9 mL (0.36 mol) O,O'-diethylthiophosphoryl chloride dropwise. After the dripping is completed, the heat preservation reaction is continued for 7 hours. After the reaction is complete, cool to room temperature, wash with water, dry the toluene layer with anhydrous sodium sulfate, filter, and desolvate to obtain brown-yellow O,O'-diethyl-2-oxo-1,3-thiazolidine-3-yl Thiophosphate.
[0030] Add the above-mentioned O, O'-diethyl-2-oxo-1,3-thiazolidine-3-ylthiophosphate (0.273mol) and 182mL (0.82mol) 33% into a 250mL four-necked flask The diethylamine aqueous solution was stirred, heated to reflux, and the reaction was kept for 30 hours. The reaction was stopped and concentrated to obtain a pale yellow viscous phosphorothioate ammonium salt. The above-mentioned phosphorothioate ammonium salt...
Embodiment 3
[0032] In a 500 mL four-necked flask, 31.2 g (0.3 mol) 2-thiazolidinone, 40.3 g (0.33 mol) 4-dimethylaminopyridine and 200 mL xylene were added, and stirred with electric power. Heat to 90°C, and add 57.9 mL (0.36 mol) O,O'-diethylthiophosphoryl chloride dropwise. After the dripping is completed, the heat preservation reaction is continued for 7 hours. After the reaction is complete, cool to room temperature, wash with water, dry the toluene layer with anhydrous sodium sulfate, filter, and desolvate to obtain brown-yellow O,O'-diethyl-2-oxo-1,3-thiazolidine-3-yl Thiophosphate.
[0033] Add the above-mentioned O, O'-diethyl-2-oxo-1,3-thiazolidine-3-yl thiophosphate (in 0.273 mol) and 82.5 mL (0.55 mol) 33 into a 250 mL four-necked flask. % Dimethylamine aqueous solution, stir, heat to reflux, keep the temperature for 30 hours and react. The reaction was stopped and concentrated to obtain a pale yellow viscous phosphorothioate ammonium salt. The above-mentioned phosphorothioate ammo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com