Process for preparing mesopored alumina
A technology of alumina and preparation steps, applied in the direction of alumina/hydroxide, etc., can solve the problems of complicated operation process, limited industrialization, toxicity, etc., and achieve the effect of low raw material, convenient control and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Example 1 Prepare boehmite sol with commercial boehmite powder.
[0023] Get 13.67g boehmite powder (Al 2 o 3 The mass content is 74.6%) was dispersed in 200ml of water, 14ml of 1M nitric acid was added at 80°C, and the temperature was maintained to continue stirring for 6 hours to obtain a stable and highly dispersed 1M boehmite sol.
example 2
[0024] Example 2 Preparation of mesoporous alumina under alkaline conditions with surfactant as template.
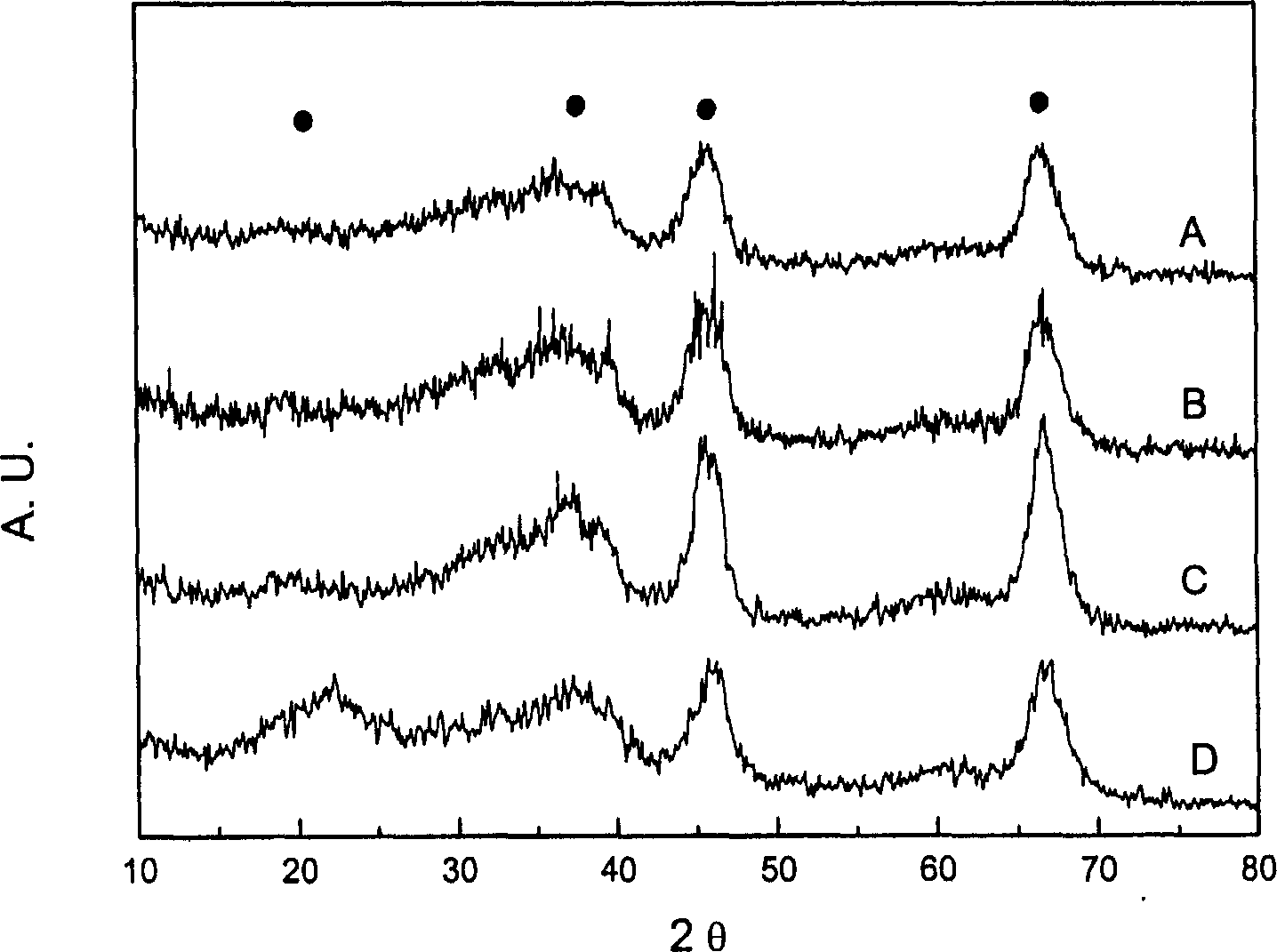
[0025] At 30°C, add 3.64g of cetyltrimethylammonium bromide (CTAB), 3.6g of urea (or 60ml of 2M ammonia water) into 40ml of 1M boehmite sol, and continue stirring for 12 hours after completely dissolving. Transferred to 100°C for hydrothermal treatment for 24 hours, the pH of the mixture after leaving the kettle was 9.0, separated, washed, dried at 30°C, and then calcined at 500°C for 3 hours. XRD analysis proves that the skeleton of the product is composed of γ-Al 2 o 3 Composition (see attached figure 1 ). Other preparation conditions using polyethylene oxide-polypropylene oxide-polyethylene oxide (P123) and Tween-80 as templates and the main physicochemical properties of the products are shown in Table 1.
[0026]
example 3
[0027] Example 3 Directly prepare mesoporous alumina with surfactant as template.
[0028] At 35°C, 5.80g of P123 was dissolved in 20ml of 1M boehmite sol, and after continuous stirring for 12 hours, it was directly dried in a vacuum oven at 75°C and calcined at 500°C for 3 hours. XRD analysis proves that the skeleton of the product is composed of γ-Al 2 o 3 Composition (see attached picture). Other preparation conditions using CTAB, bis(2-ethylhexyl)sodium sulfosuccinate (AOT), and Tween-80 as templates and the main physicochemical properties of the products are shown in Table 2.
[0029]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com