Process for synthesizing stable sodium azulene sulfonate
A technology for the synthesis of sodium azulene sulfonate, which is applied in the field of stable sodium azulene sulfonate synthesis technology, can solve the problems of waste acid and organic solvent generation, high toxicity of dioxane, environmental pollution, etc., and achieve pollution reduction, The effect of solving the problem of toxic work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
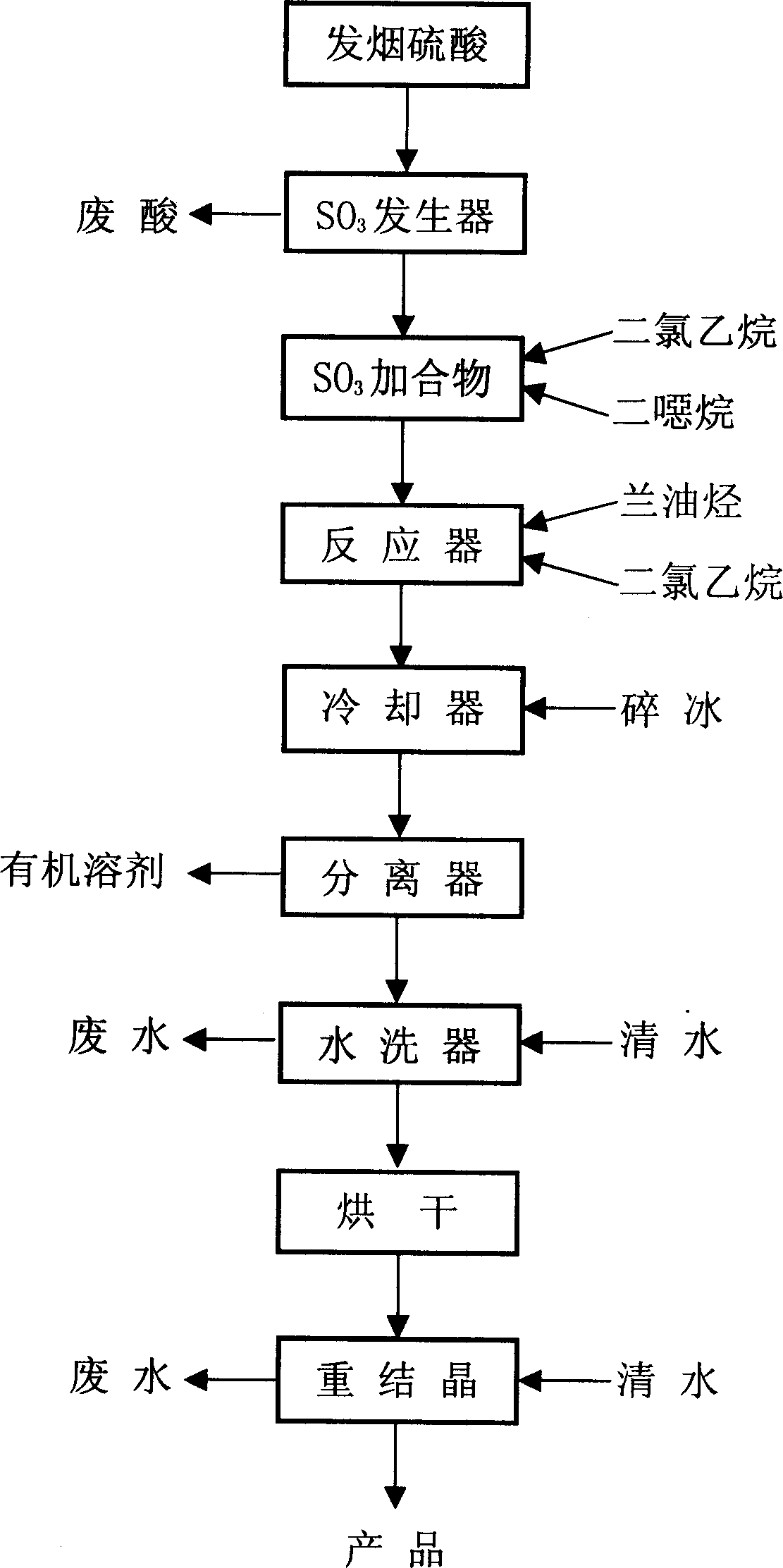
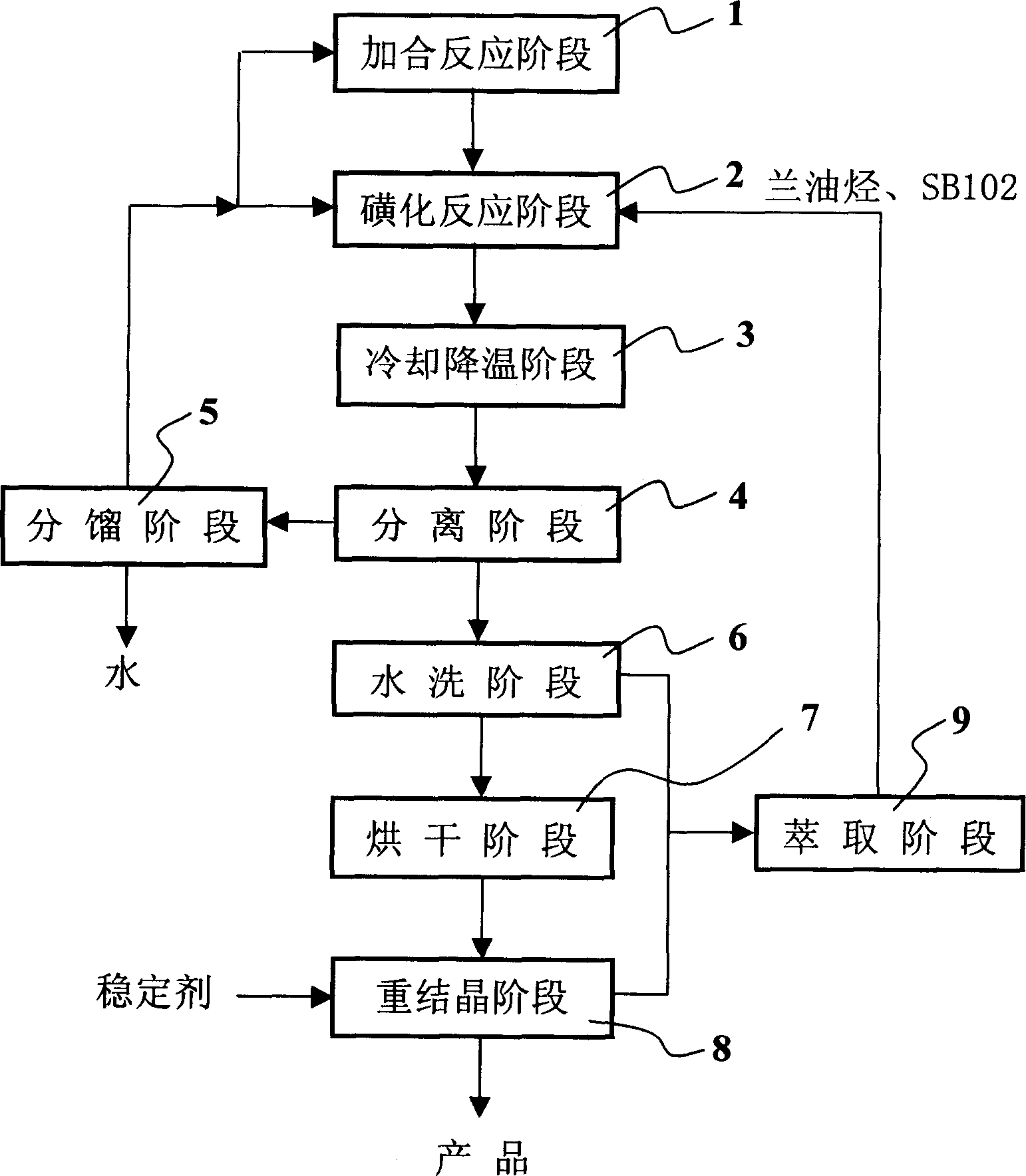
[0027] Add 300 ml of dichloroethane and 85 g of SB102 reagent into a 500 ml three-necked flask, feed in liquefied sulfur trioxide, control the temperature at 0°C, stop feeding after the weight increases to 80 g, and continue stirring for 20 minutes for later use .
[0028] Add the reagent obtained above dropwise to a solution container containing 100 grams of lanoleic hydrocarbon and 250 milliliters of dichloroethane at 0°C under stirring. After the addition, raise the temperature to 20°C and continue stirring for 30 minutes. In a container with 1000 grams of crushed ice, let it stand until the crushed ice melts, separate the organic layer and reuse it after fractional distillation. The aqueous layer was adjusted to PH=8 with sodium carbonate, washed with water and dried at 40°C for 3 hours, dissolved in water and added with 0.6 g of a stabilizer, recrystallized to obtain 120 g of flaky crystals, yield 75%, The water after washing and crystallization was extracted with 300 ml...
Embodiment 2
[0030] Add 200 ml of dichloroethane and 60 g of SB102 reagent into a 500 ml three-necked flask, feed in liquefied sulfur trioxide, control the temperature below 0°C, stop feeding when the weight increases to 55 g, and continue stirring for 20 minutes first stand-by.
[0031] The reagent obtained above was added dropwise to a solution of 67 g of lanoleic hydrocarbon and 170 ml of dichloroethane at 0° C. with stirring, and stirred at 20° C. for 30 minutes after the addition was completed. Transfer to the container that 700 grams of crushed ice is housed, stand until the crushed ice melts, separate the organic layer and reuse it after fractional distillation. The water layer was adjusted to PH=8 with sodium carbonate, washed with water, and dried at 80°C for 3 hours. The obtained product was dissolved in water and 0.4 g of stabilizer was added to recrystallize to obtain 78 g of flaky crystals, with a yield of 73%. Washed with water And crystallized water, use 200 milliliters of ...
Embodiment 3
[0033] Add 250 ml of dichloroethane and 75 g of SB102 reagent into a 500 ml three-necked flask, feed in liquefied sulfur trioxide, control the temperature below 0°C, stop feeding when the weight increases to 70 g, and continue stirring for 20 minutes first stand-by.
[0034] The reagent obtained above was added dropwise to a solution of 85 g of lanoleic hydrocarbon and 210 ml of dichloroethane at 0° C. with stirring, and stirred at 20° C. for 30 minutes after the addition was completed. Go to the container that 850 grams of crushed ice are housed, let stand until the crushed ice melts, separate the organic layer and reuse it after fractional distillation. The aqueous layer was adjusted to PH=8 with sodium carbonate, washed with water and dried at 80°C for 3 hours, dissolved in water and added with 0.5 g of a stabilizer, recrystallized to obtain 98 g of flaky crystals, yield 74%, The water after washing and crystallization was extracted with 250 ml of dichloroethane and SB102 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com