Method for purifying virus
A virus and anion technology, applied in the field of virus purification, can solve the problems of difficult to scale-up industrial-scale production, time-consuming, expensive and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] Preparation of cell lysates
[0067] Virus can be purified from cell lysates prepared from a number of sources mentioned above, including cell lines, tissues, body fluids, organs, and the like. Viruses are often purified from cell lysate preparations prepared from virus-infected cells, where the cells have been cultured using cell culture methods. For example, adenovirus can be isolated from virus-infected cells such as 293 cells, HeLa cells, and the like. Cells can be infected at a high multiplicity of infection (MOI) to optimize yield.
[0068] Virus-containing cell lysates can be prepared by any method suitable for the release of virus from infected cells. Virus can be released from infected cells using techniques known in the art or by autolysis. Preferred methods of lysing virus-infected cells include the use of hypotonic solutions, hypertonic solutions, sonication, pressure, or detergents. A preferred technique is the use of detergents, more preferably a combi...
Embodiment 1
[0086] Purification of Adenovirus
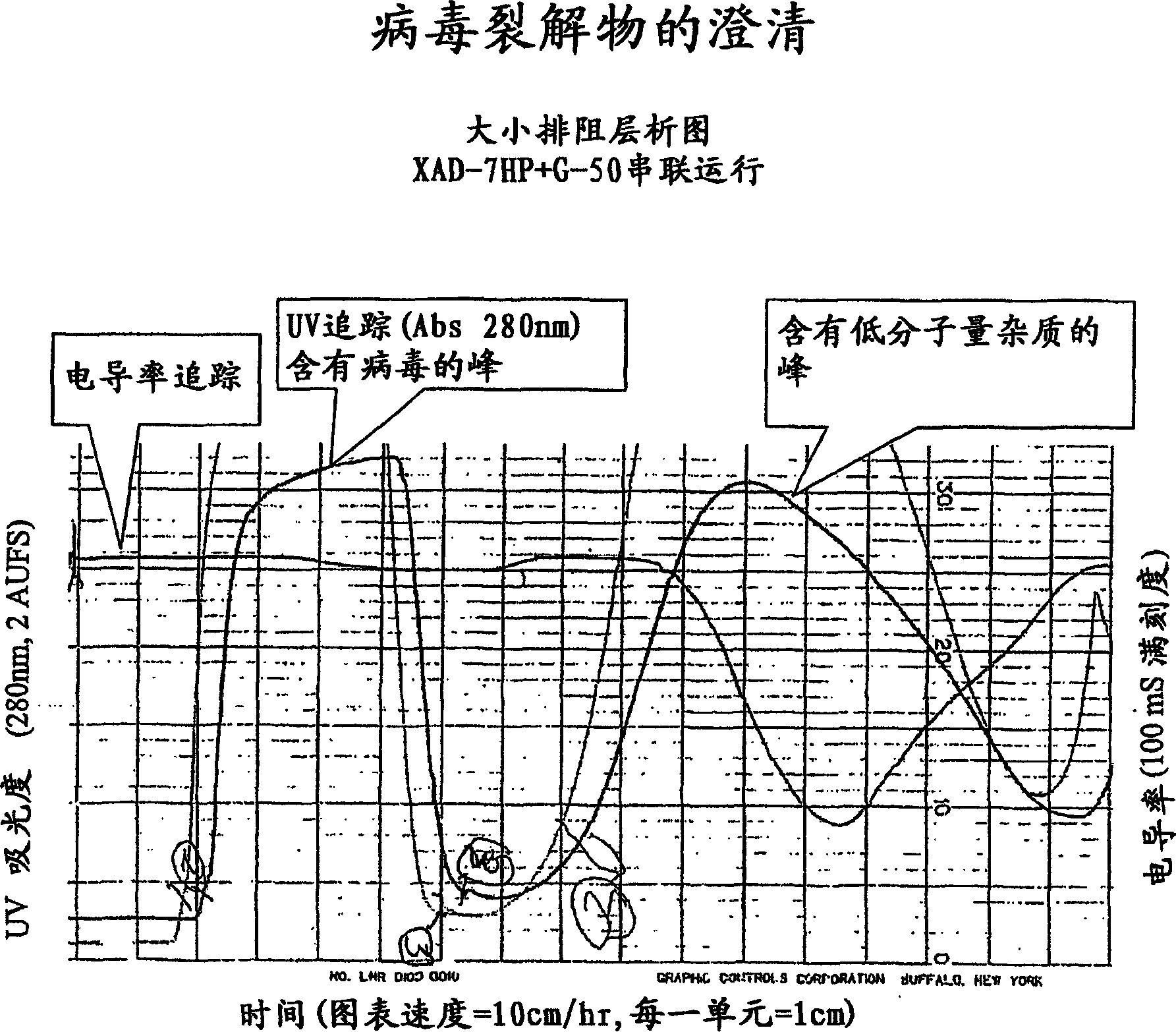
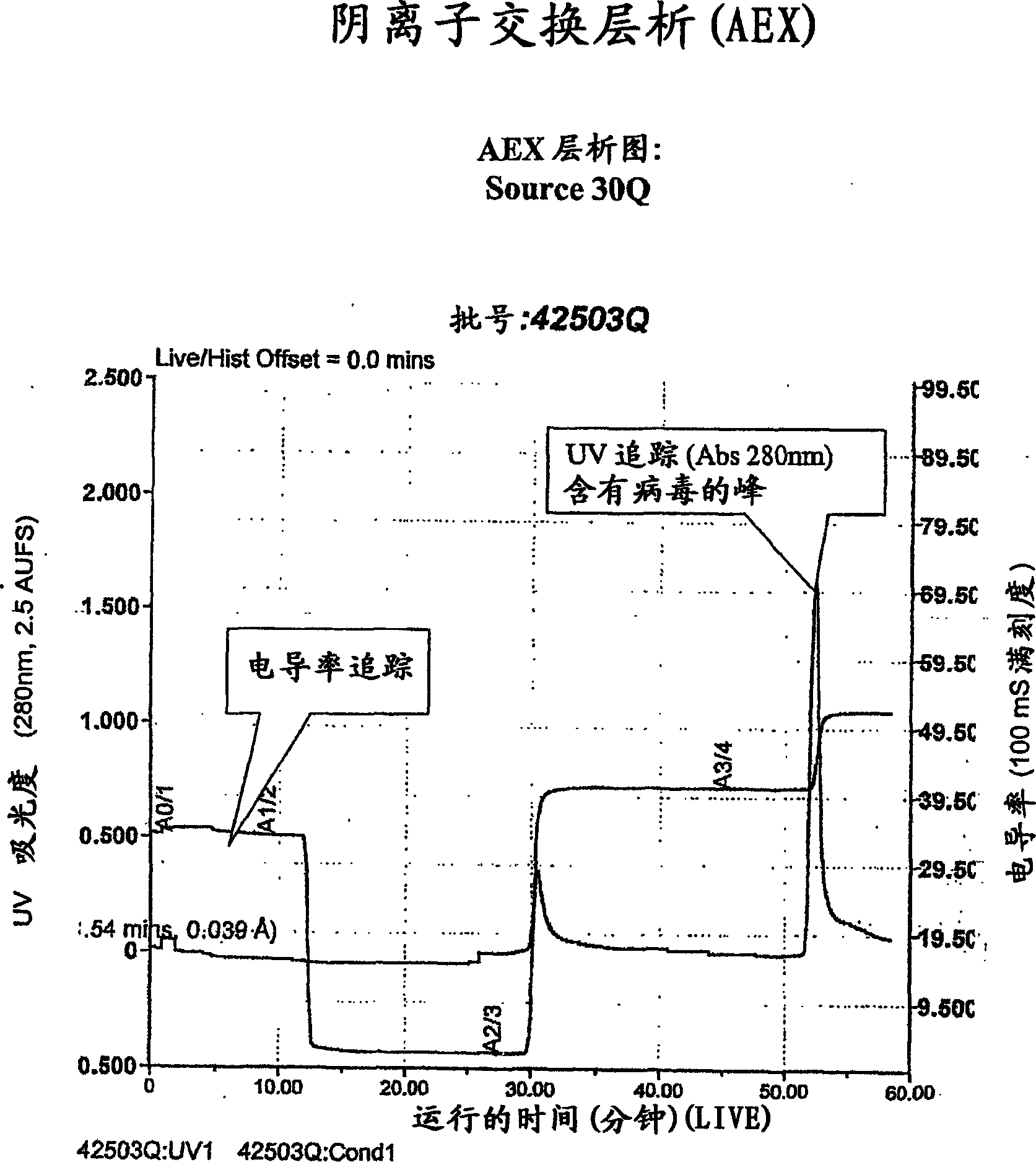
[0087] figure 1 A general purification scheme is shown. Viruses were monitored at certain steps of the purification process described in this example. The method involves measuring virus concentrations by AEX-HPLC similar to that described in Huyghe, et al, Human Gene Therapy 6: 1403-1416 (November 1995). In summary, a 1 ml Resource-Q column was equilibrated with a buffer containing 300 mM NaCl and 20 mM phosphate buffer pH 7.5. Virus was eluted by running a linear gradient of 300 mM → 600 mM NaCl and monitoring the UV absorbance at 260 and 300 nm. The areas of the virus peaks were integrated and compared to a cesium chloride purified reference standard.
[0088] Preparation of cell lysates
[0089] Hela-S3 cells, obtainable from American Type Culture Collection, Accession No. ATCC CCL-2.2, were infected with adenovirus Onyx 411 as described by Johnson, et al., Cancer Cell. 2002 May;1(4):325-37 . 70 liters of cell culture harvest was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com