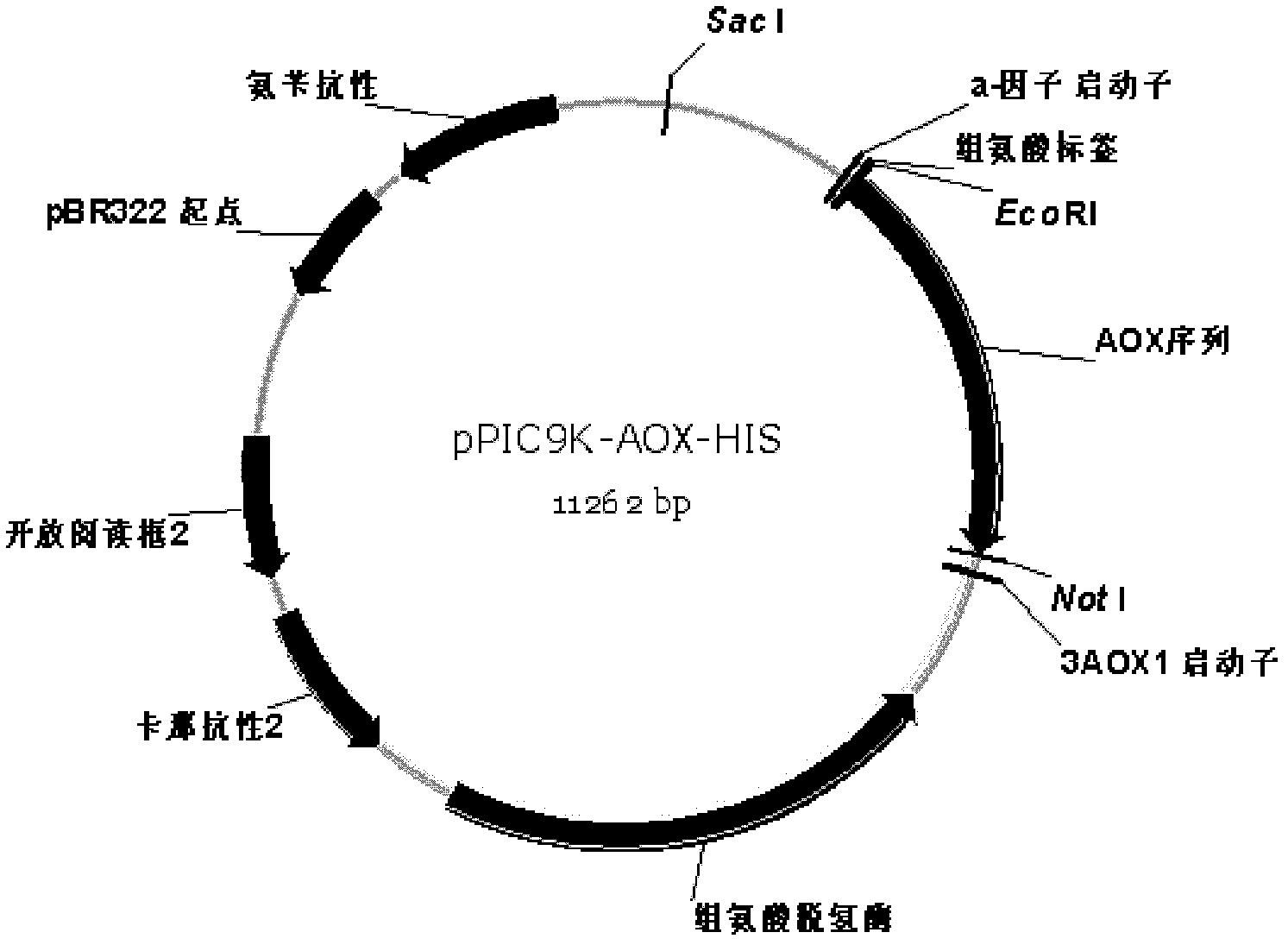
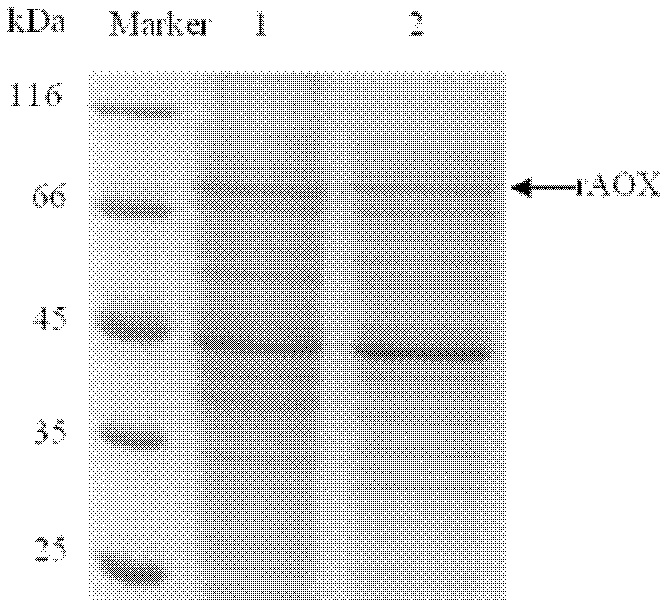
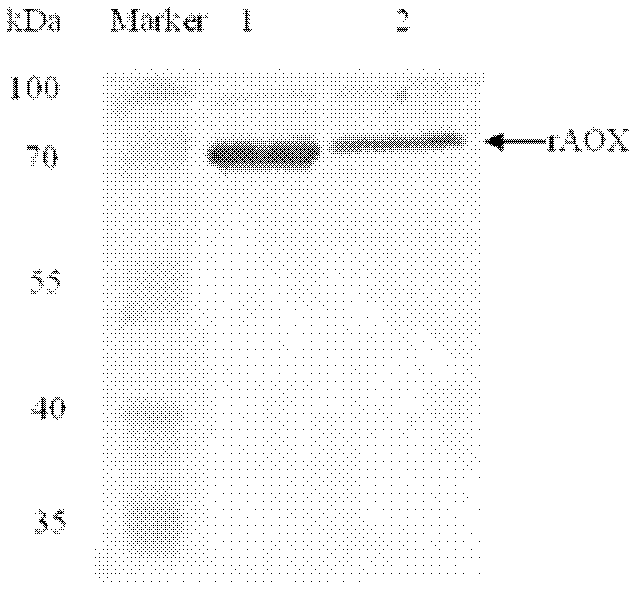
Method for expressing and purifying recombinant ethanol oxidase in pichia pastoris
A technology of alcohol oxidase and alcohol oxidase protein, which is applied in the direction of oxidoreductase, biochemical equipment and methods, recombinant DNA technology, etc., can solve the problem of enzyme inactivity, etc., and achieve increased expression, optimized fermentation conditions, and enzyme purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The present invention is further described by steps below:
[0033] Step 1, Pichia pastoris genome extraction method:
[0034] 1) Inoculate Pichia pastoris GS115 to 5ml yeast extract peptone glucose (YPD) medium, culture at 30°C for 16-18 hours, take 1.5ml of yeast culture solution, and centrifuge at 5000 rpm for 5-10 minutes at room temperature to collect the bacteria ;
[0035] 2) The cells were washed once with sterile water and centrifuged at 5000 rpm for 5 minutes; the cells were resuspended in 200 μL TE buffer, and 30 μL 10 mg / ml lysozyme was added to incubate at 37°C for 1 hour; 100 μL 2% dodecyl Mix sodium sulfate (SDS), freeze at -20°C, then shake at room temperature to dissolve, repeat 3 times; add 150 μL 5mol / L potassium acetate with pH 8.9 to the suspension, mix gently, and then centrifuge at 10,000 rpm for 5 minute;
[0036] 3) Transfer the supernatant to a new 1.5ml centrifuge tube, add 2 times the volume of ethanol, mix gently, leave at room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com