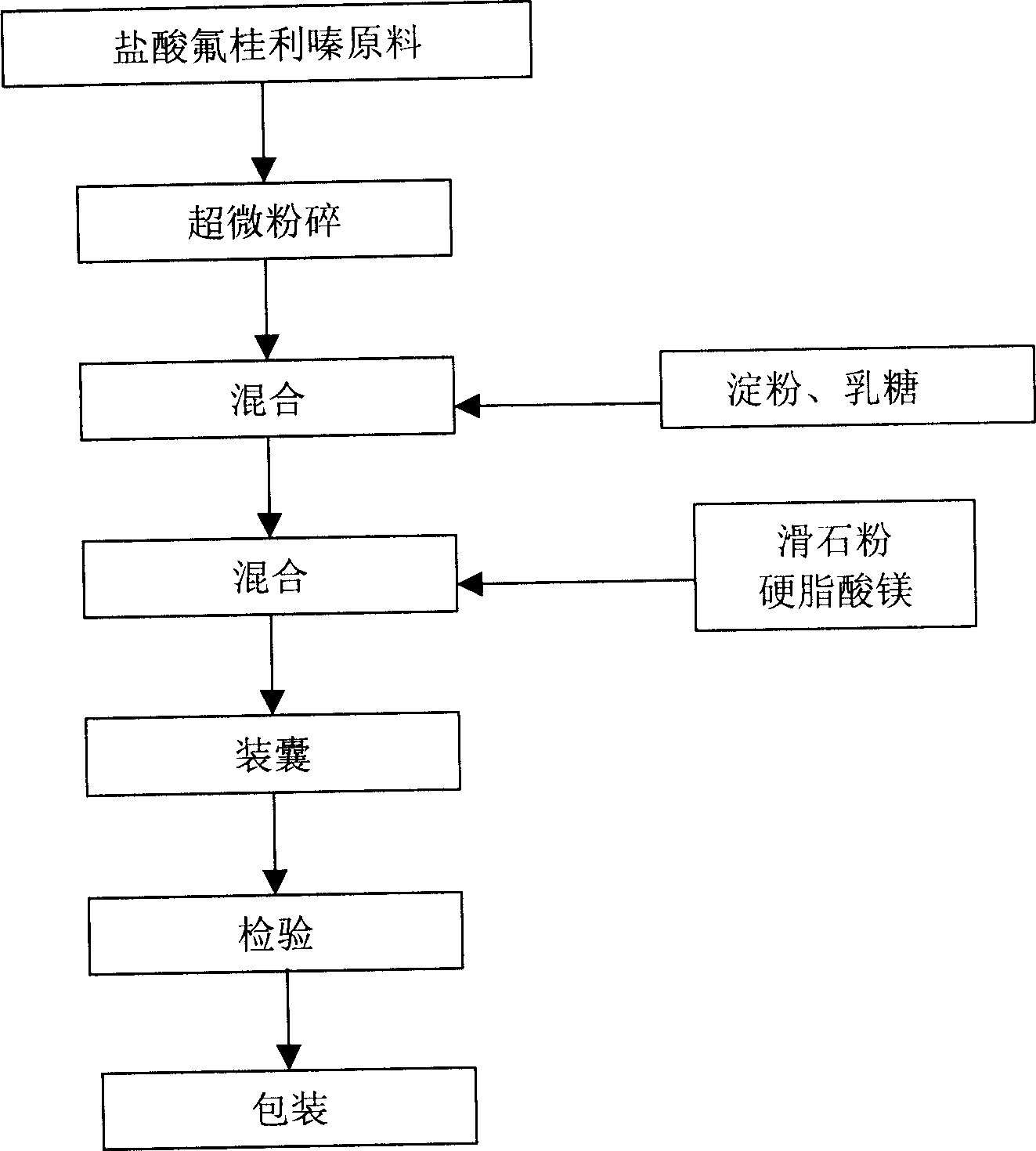
Flunarizine hydrochloride capsule and its preparation method
A technology of flunarizine hydrochloride and capsules, which is applied in the field of flunarizine hydrochloride capsules and its preparation, and can solve problems affecting bioavailability and curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Get 10 kilograms of superfine powder, 15 kilograms of starch, 25 kilograms of lactose, 15 kilograms of talcum powder, 0.5 kilogram of magnesium stearate. 10 kg of the superfine powder is firstly mixed with 15 kg of starch and 25 kg of lactose, then mixed with 15 kg of talcum powder and 0.5 kg of magnesium stearate, and finally packed into capsules to be prepared.
Embodiment 2
[0024] Get 5 kilograms of superfine powder, 10 kilograms of starch, 20 kilograms of lactose, 10 kilograms of talcum powder, 0.2 kilogram of magnesium stearate. 5 kg of the superfine powder is firstly mixed with 10 kg of starch and 20 kg of lactose, then mixed with 10 kg of talcum powder and 0.2 kg of magnesium stearate, and finally packed into capsules.
Embodiment 3
[0026] Get 20 kilograms of superfine powder, 20 kilograms of starch, 30 kilograms of lactose, 20 kilograms of talcum powder, 0.8 kilogram of magnesium stearate. 20 kg of the superfine powder is firstly mixed with 20 kg of starch and 30 kg of lactose, then mixed with 20 kg of talcum powder and 0.8 kg of magnesium stearate, and finally packed into capsules to be prepared.
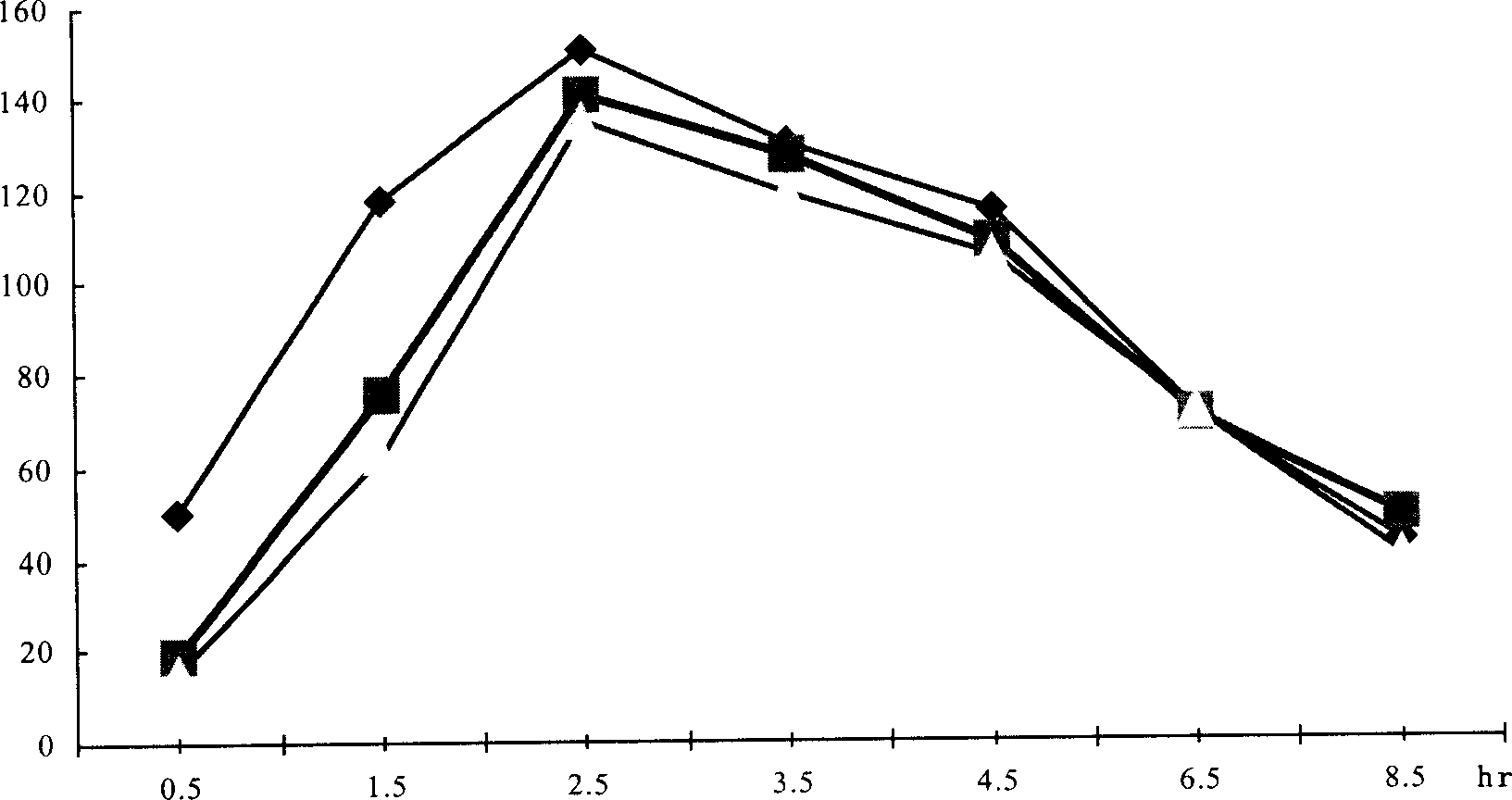
[0027] Can be reflected by comparative bioavailability. The following compares the bioavailability of the flunarizine hydrochloride capsules produced by the two production processes through the bioequivalence test, that is, comparing the flunarizine hydrochloride capsules produced by the applicant with the ultrafine pulverization production process and the applicant's non-ultrafine production process. The human bioavailability of flunarizine hydrochloride capsules produced by crushing process was compared with that of flunarizine hydrochloride capsules produced by Shandong Fangming Pharmaceutical Co. Cinnari...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com