Refining method for copper chloride etching waste liquid and refined copper chloride solution
A technology of etching waste liquid and refining method, applied in copper chloride, chemical instruments and methods, copper halide and other directions, can solve the problems of high operation cost and complicated operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
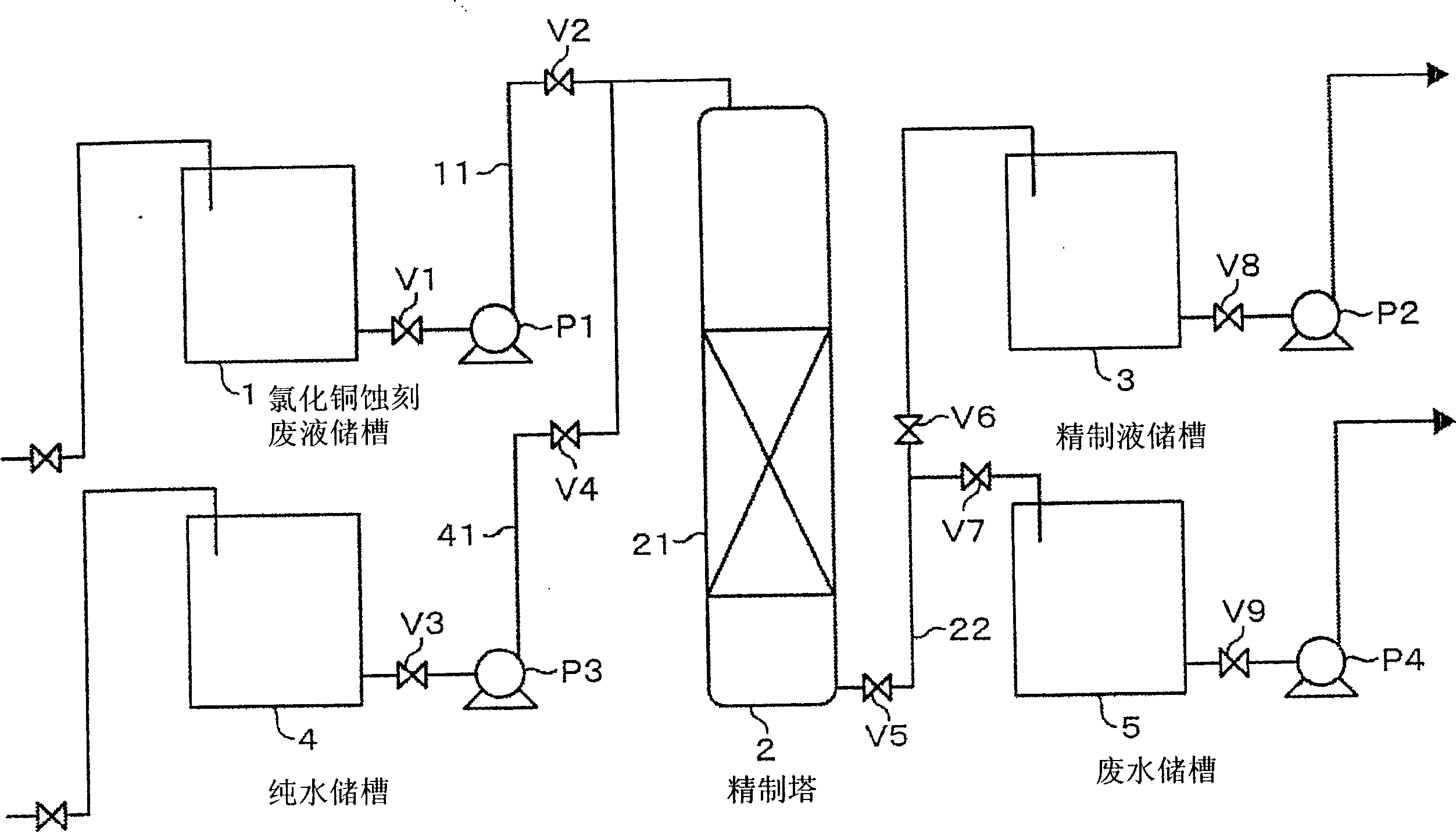
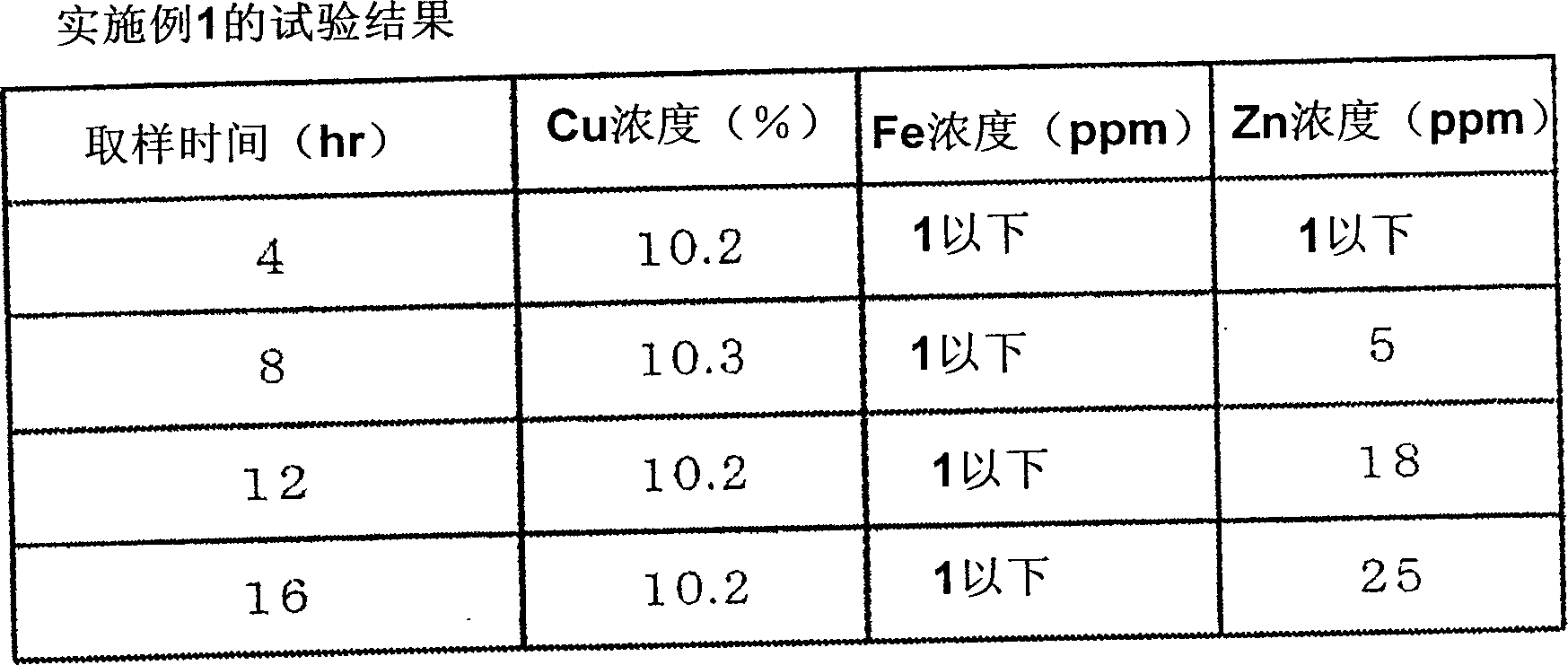
[0048] As the anion exchange resin, a 100 mL portion of strongly basic anion exchange resin "DIAION (registered trademark) PA316" produced by Mitsubishi Chemical Corporation was packed in the column, and the column was filled at SV=1.5 hr. -1 Make the copper chloride etching waste liquid pass through. The so-called SV is an abbreviation for Space Velocity. The composition of the copper chloride etching waste liquid had a Cu concentration of 10.2%, a free HCl concentration of 7.6%, a Fe concentration of 320 ppm, a Zn concentration of 40 ppm, and a pH of -1.3. In addition, the liquid temperature is controlled at 60±2°C. figure 2 Indicates the result of analyzing the liquid discharged from the column.
Embodiment 2
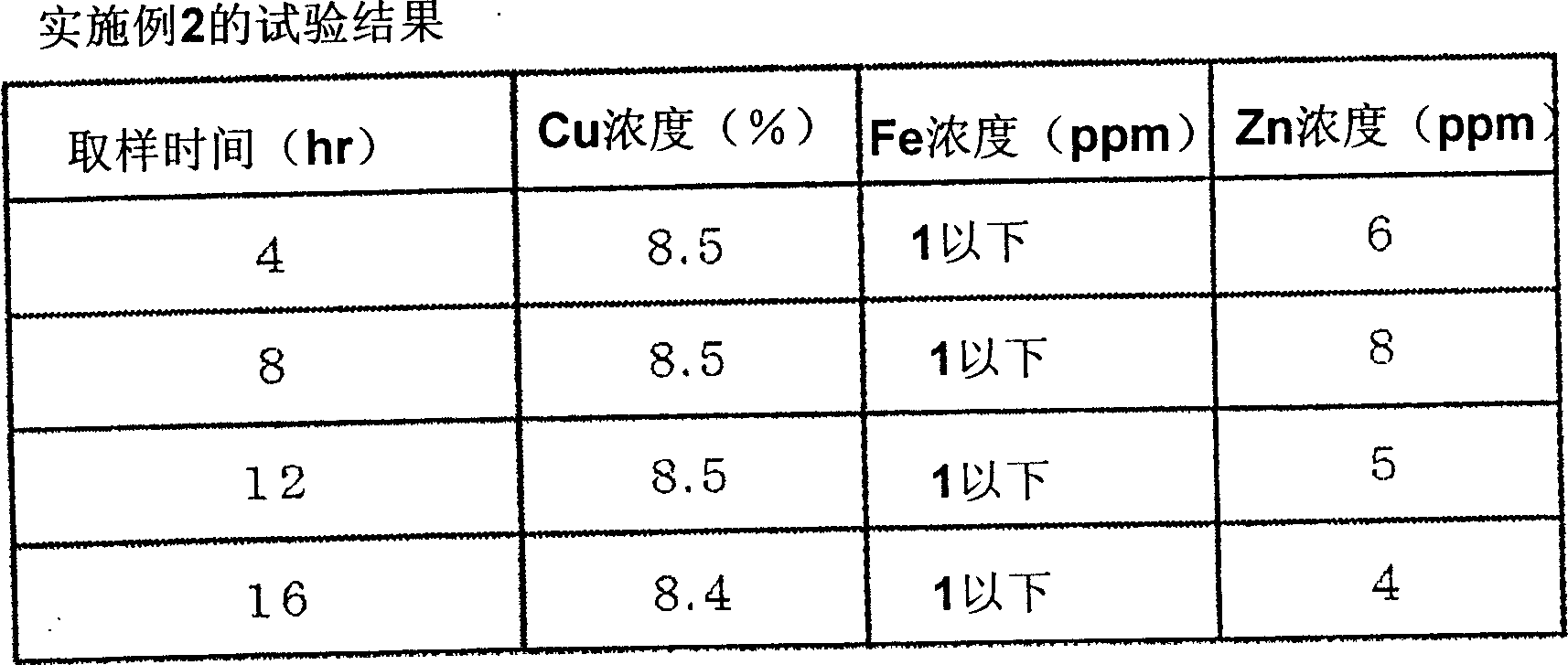
[0050] In addition to the composition of the copper chloride etching waste liquid, the concentration of Cu is 8.6%, the concentration of free HCl is 1.5%, the concentration of NaCl is 9.3%, the concentration of Fe is 5ppm, the concentration of Zn is 43ppm, the pH is -0.5, and the temperature of the liquid is controlled at Except for 20±2°C, the test was performed in the same manner as in Example 1. image 3 Indicates the result of analyzing the liquid discharged from the column.
Embodiment 3
[0052] As the anion exchange resin, the same resin as in Example 1 was used, and the column was packed in 50 mL portions at SV=1 hr -1 Make the copper chloride etching waste liquid pass through. The composition of copper chloride etching waste liquid, the concentration of Cu is 8.1%, the concentration of free HCl is less than 1%, the concentration of NaCl is 9.2%, the concentration of Fe is 48ppm, the concentration of Zn is 25ppm, the pH value of the liquid is +1.0, and the temperature of the liquid is controlled At 40±2°C. Figure 4 Indicates the result of analyzing the liquid discharged from the column.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com