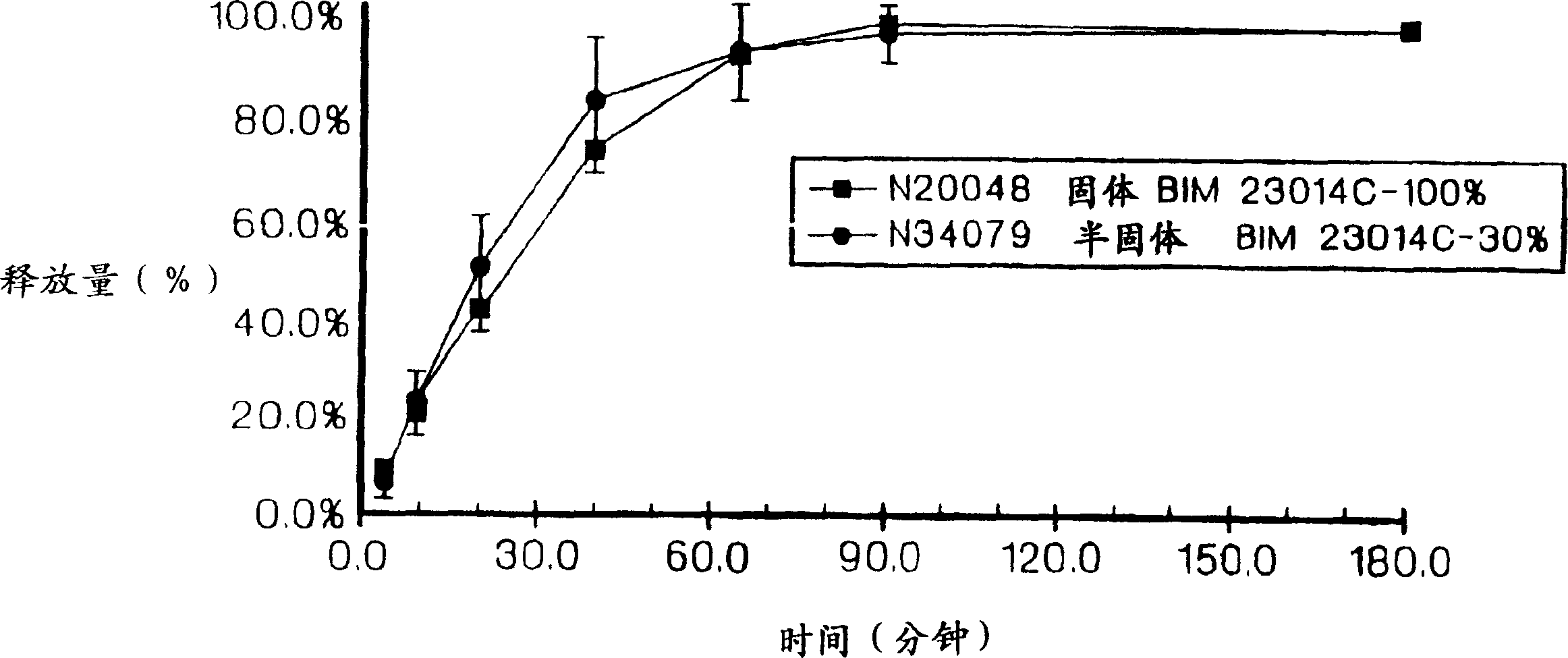
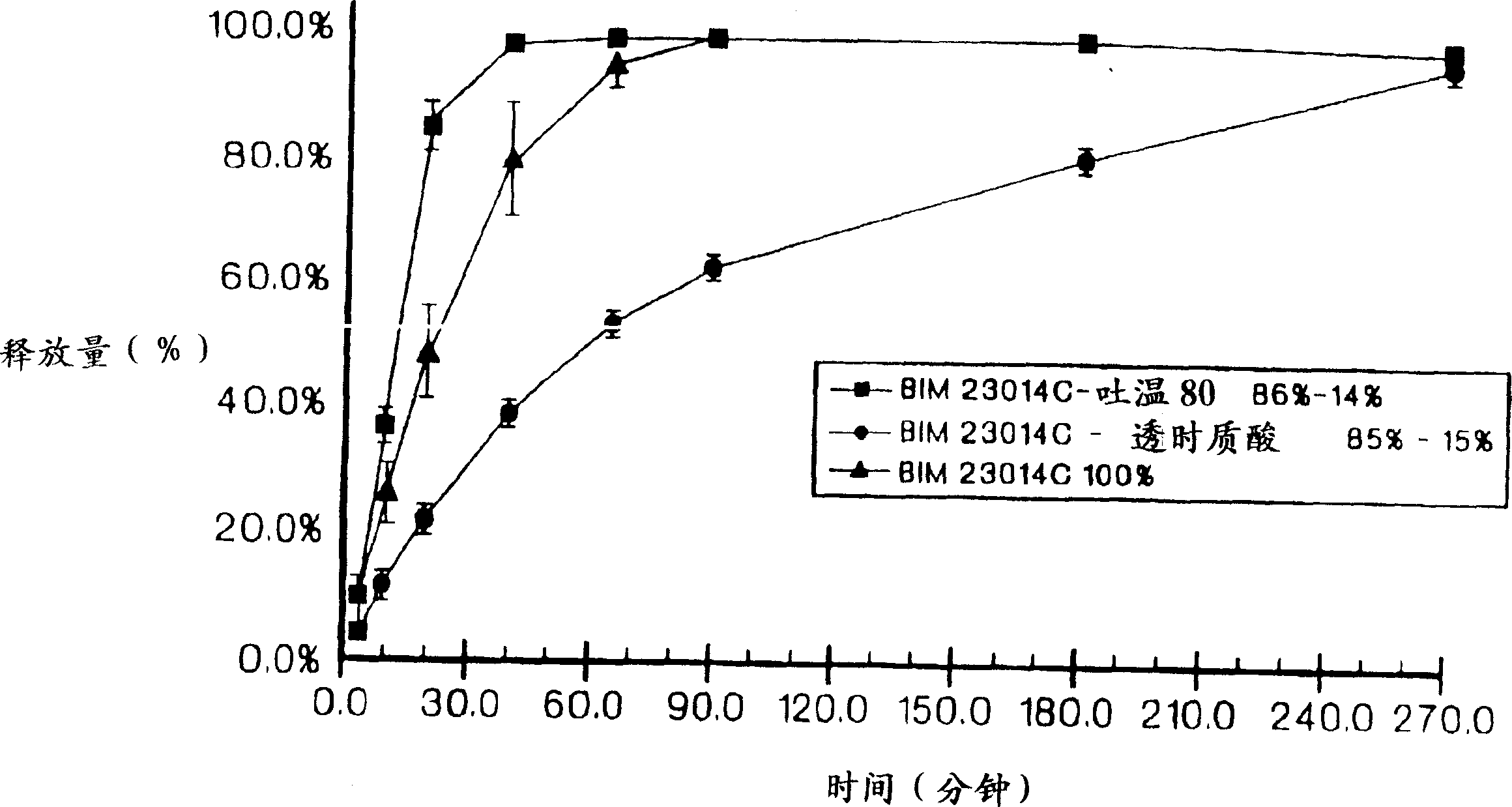
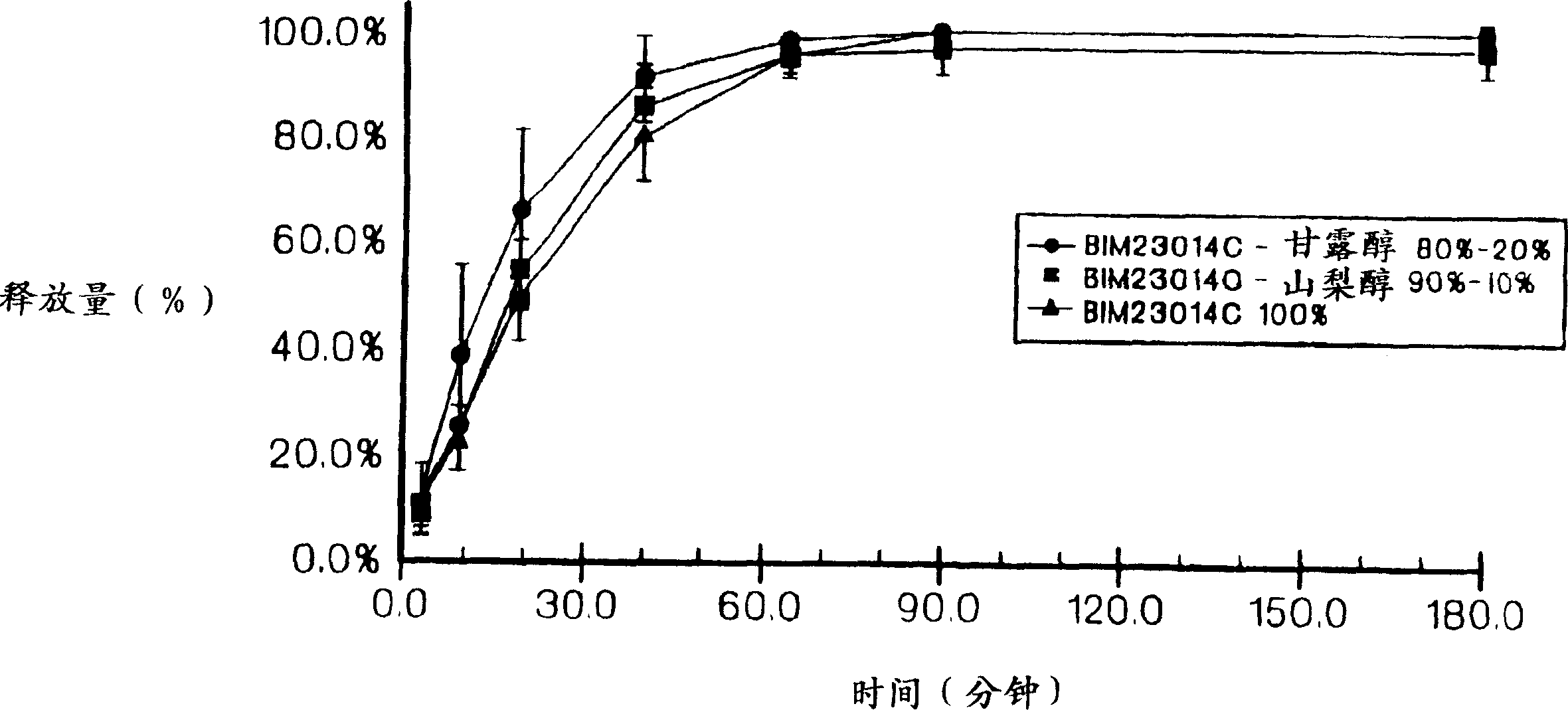
Sustained release of peptides from pharmaceutical compositions
A composition and drug technology, applied in the direction of drug devices, drug delivery, medical containers, etc., can solve problems such as discomfort and patient inconvenience, and achieve the effect of reducing volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] The preparation method of the present invention avoids the dissolution problems of many peptides because it is not necessary to dissolve the peptides prior to injection. Another advantage of the solid composition of the present invention is its stability. Anhydrous solid compositions avoid problems of degradation, crystallization, bulking and solidification associated with hydration sustained release formulations such as hydrogels.
[0079] The following is a method of mixing the peptide and the carrier, and filling the resulting solid pharmaceutical composition into a syringe through a trocar.
[0080] The carrier, such as sorbitol, is dissolved in a liquid excipient for manufacture, such as water or an organic solvent, and the resulting solution is mixed with the desired peptide to form a homogeneous semisolid mixture. If the final solid composition does not contain a carrier, the peptide is simply mixed with water or other liquid excipient to form a semi-solid mixtu...
Embodiment 1
[0094] Example 1: 100% SOMATULINE TM solid composition
[0095] Add 140 mg of water to the somatostatin analog SOMATULINE TM (Kinerton, Ltd., Dublin, Ireland) acetate 60 mg. Unless otherwise specified, SOMATULINE in the following columns TM In the example, SOMATULINE is used TM of acetate. The mixture was kneaded with a spatula in a 2 ml plastic syringe and added to a stainless steel syringe having a 5 mm internal diameter chamber and a 2.5 mm internal diameter extrusion tip. The mixture was extruded through a syringe under the action of a syringe pump to form filaments, which were cut into 3 cm sticks and collected on glass slides. The sticks were vacuum-dried for 24 hours, resulting in sticks with a diameter of 1.4 mm containing 10 mg of SOMATULINE TM / cm. Fit the small rod into a trocar with an inner diameter of 1.5 mm and a length of 3 cm.
Embodiment 2
[0096] Example 2: 80% SOMATULINE TM , a solid composition of 20% mannitol
[0097] Referring to the procedure of Example 1, firstly, 1 gram of mannitol (Roquette, Lestrein, France) was mixed with 9 grams of water to form a solution. Add 0.140 g of this solution to 0.060 g of SOMATULINE TM , the extruded filaments were sheared, collected on glass slides, and dried under vacuum for 24 hours. The resulting 2.9 cm sticks contained 40 mg of SOMATULINE TM (20% by weight mannitol and 80% by weight SOMATULINE TM ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com