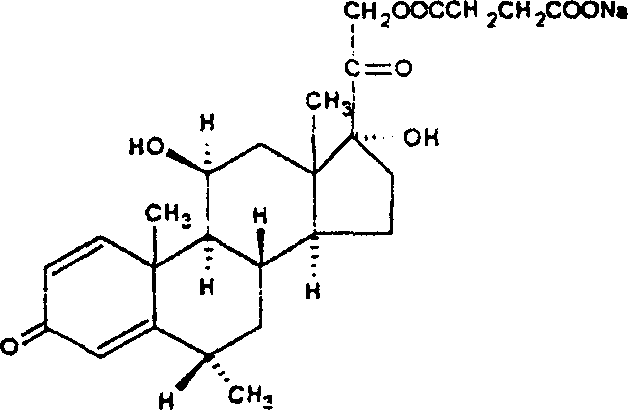
Methylprednisolone sodium succinate lyophilized composition and its preparing method
A technology of methylprednisolone sodium succinate and methylprednisolone succinate, which is applied to the freeze-dried composition containing methylprednisolone sodium succinate and the preparation field thereof, can solve the problem of easy moisture absorption, high production cost, and inability to add pH Buffers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] ①Preparation of liquid medicine: take the prescribed amount of alkaline drug excipients and dissolve them with water for injection, take the prescribed amount of pH buffer and dissolve them with water for injections, take the stabilizer and dissolve them with water for injections, accurately weigh the prescribed amount of methylprednisolone succinate, Make a paste with water for injection, slowly add alkaline solution, and stir while adding until methylprednisolone succinate is completely dissolved, then add pH buffer solution and stabilizer solution, and add water for injection to the full amount. Add medicinal charcoal, stir well, let it stand for about 20 minutes, decarburize by coarse filtration, and finely filter until the clarity of the liquid medicine is qualified. Take samples, measure and adjust the pH value of the medicinal solution to 7-8. Sterilize and filter with a Φ0.22 μm microporous membrane filter. Sampling and determination shows that the prope...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com