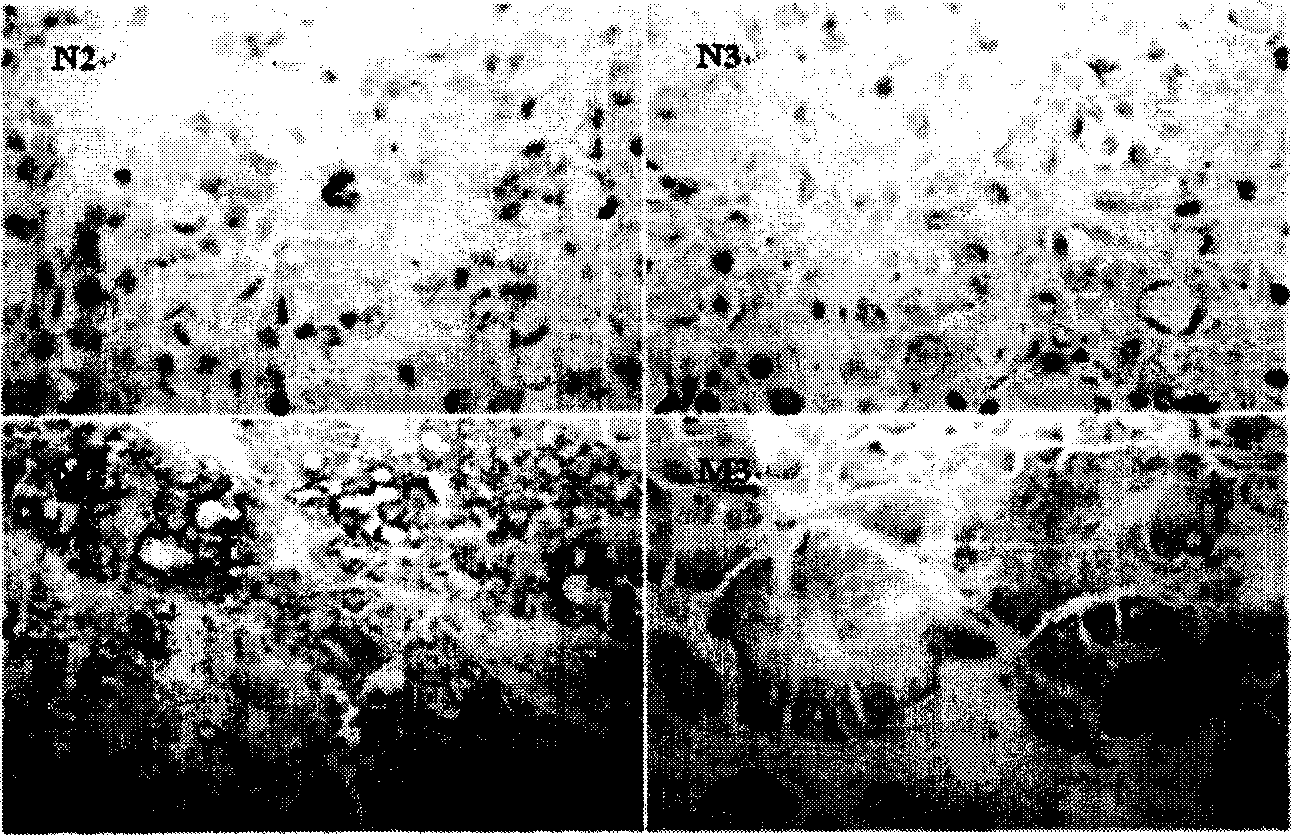Pharmaceutical use of heat shock protein 27 for serological identification and liver cancer diagnosis
A technology for heat shock protein and serum preparation, applied in biological testing, scientific instruments, pharmaceutical formulations, etc., can solve problems such as prediction and judgment of liver cancer recurrence and metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Using conventional methods in this field, the full protein expression profiles of LCID20 and LCID35 in nude mouse models of human liver cancer with different metastatic potentials were compared and studied: the number of proteins displayed by 2D electrophoresis was as high as 2500-3000, and the analysis and verification of differential proteins were carried out. High expression of HSP27 was found.
Embodiment 2
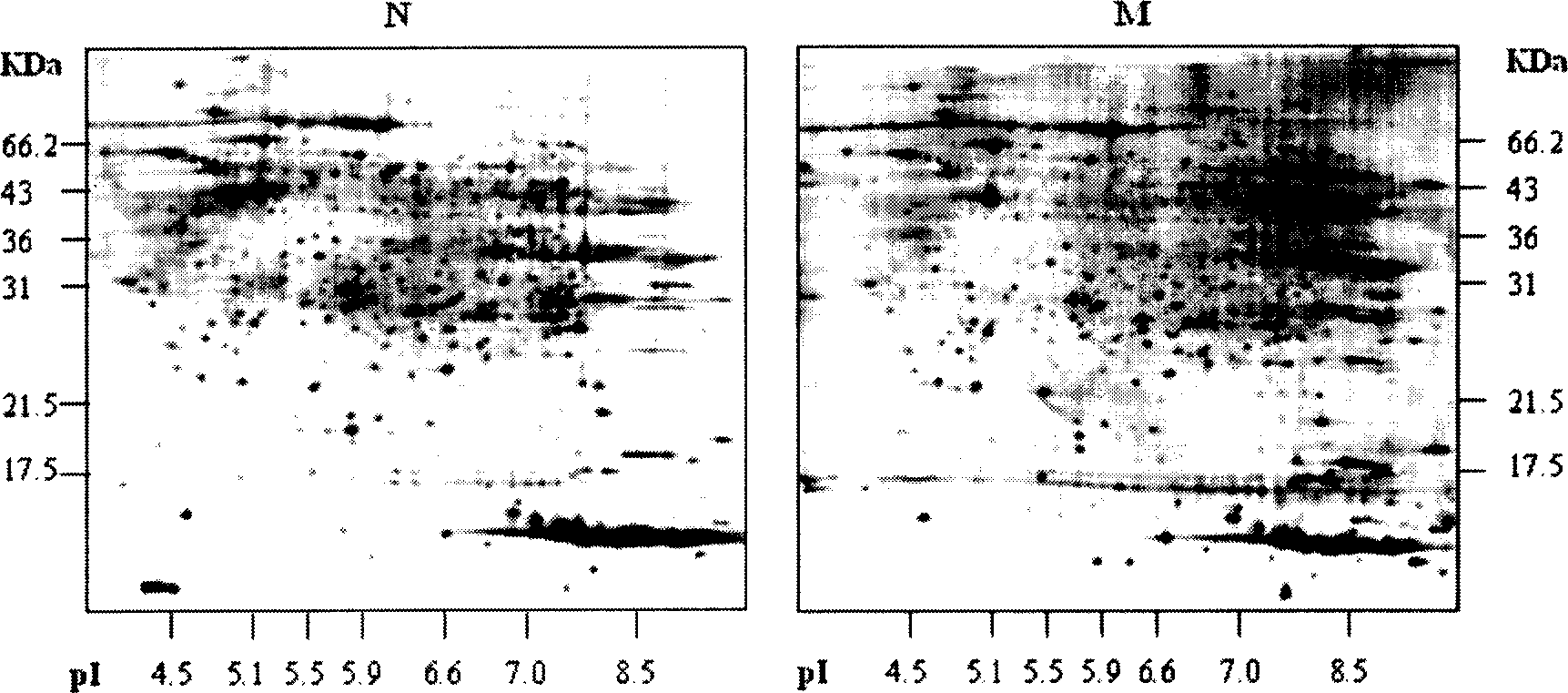
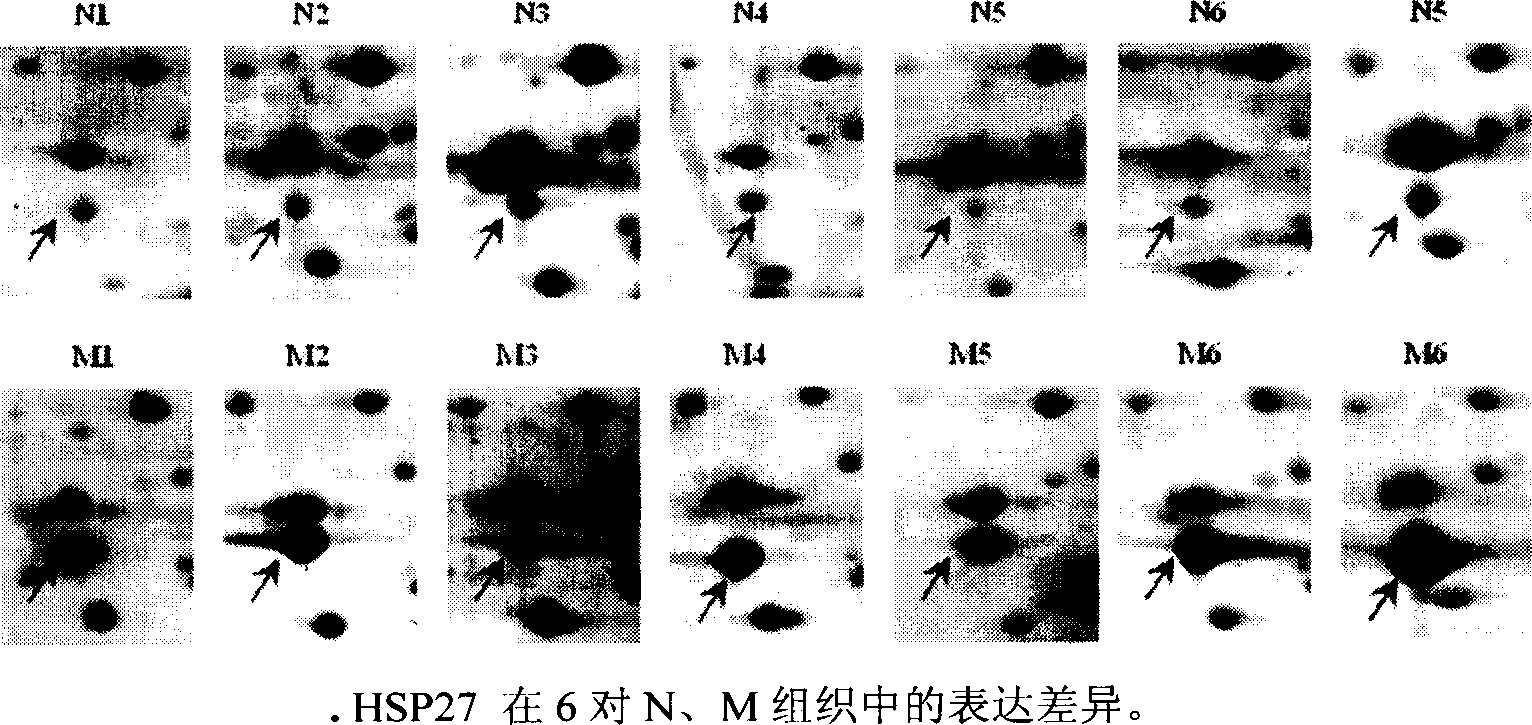
[0026] Two-dimensional gel electrophoresis (2-DE) was used to separate the proteins of 6 HCC tissues with clinical and pathological metastasis and 6 HCC tissues without metastasis, and the similarities and differences between the 2-DE patterns of the two groups were analyzed using Image Master software 16 protein spots with significant differences were identified by MALDI-TOF-MS and database search. The results showed that these proteins were S100 calcium-binding protein (S100), 27KDa heat shock protein (HSP27), keratin 18 (CK18), etc., which were related to cell movement, signal transduction, energy metabolism, etc. respectively. The expression of HSP27 in 6 cases of metastatic HCC tissues was significantly higher than that in 6 cases of non-metastatic HCC tissues, and the results of 2-DE were further verified by western blotting analysis. There were also differences in mRNA levels between the two groups detected by RT-PCR, but Not significant, immunohistochemical detection s...
Embodiment 3
[0028] Carry out serum proteomics research, on the 2DE map of serum protein after removing high-abundance albumin and globulin, use two-dimensional electrophoresis combined with Maldi-TOF-MS method to compare and study normal people, HBV patients and liver cancer patients Differential expression of proteins, found at least 8 kinds of differential proteins, Transferrin (transferrin, TF), Transthyretin (TTR), α1-antitrypsin (antitrypsin α1), Clusterin (CLU), Haptoglobinchain (hepatoglobulin α2 chain , HP), Ceruloplasmin (CP), Heat shockprotein 27 (heat shock protein 27, HSP27), alpha-fetoprotein (alpha-fetoprotein); semi-quantitatively found that HSP27 is unique to liver cancer.
[0029]The results of Western Blot identification of 60 cases of serum found that no HSP27 was found in normal human serum (0 / 20), and only one case of HBV patients found HSP27 (95%, 1 / 20); all 20 cases of HCC patients had strong HSP27 in serum (100%, 20 / 20). It suggested that HSP27 could be used in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com