Selectable ion concentrations with electrolytic ion exchange
A technology of ion concentration and cation exchange, which is used in the field of processing influent liquid containing ions, which can solve the problems of uncontrollable ion concentration and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Constant Voltage
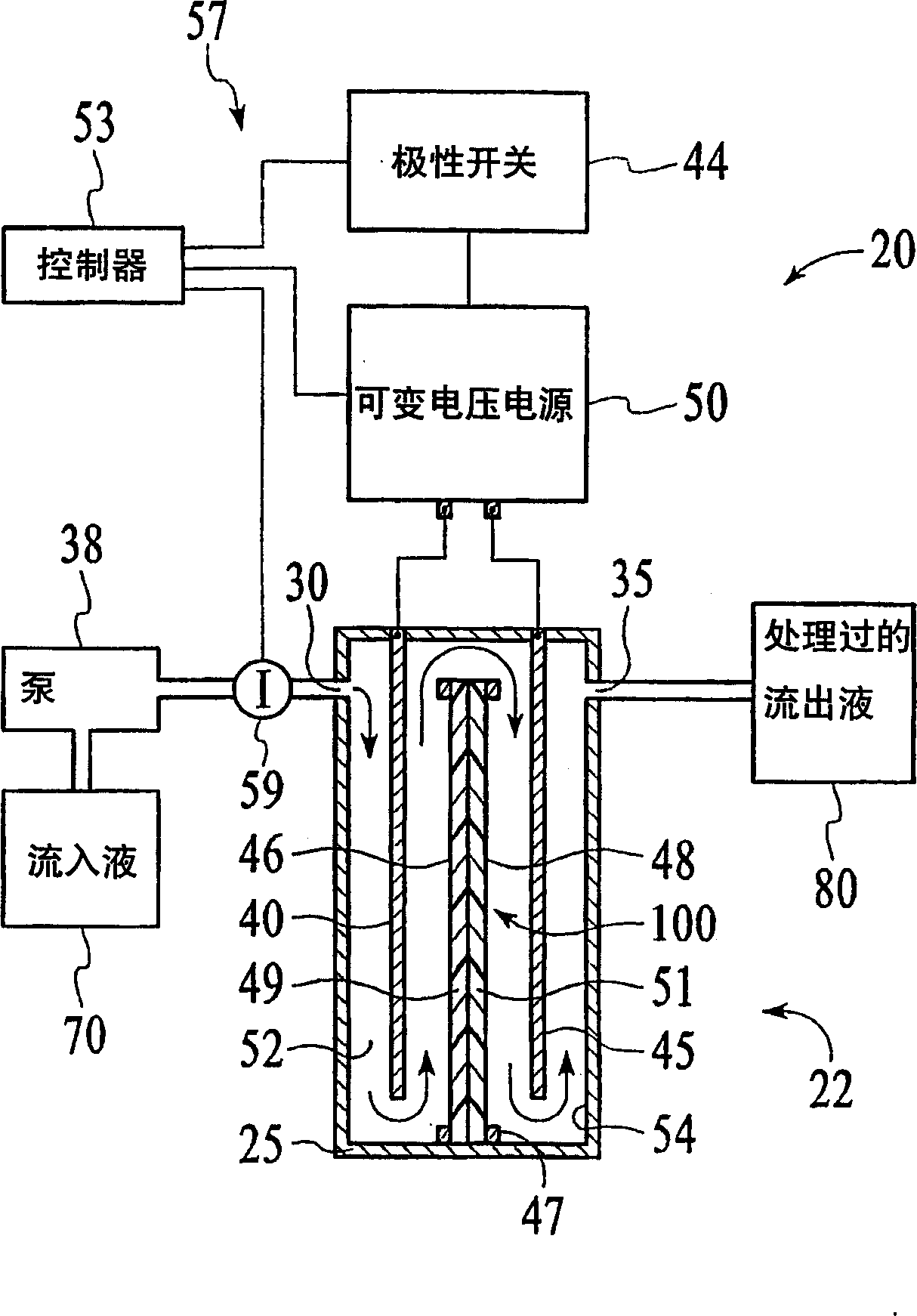
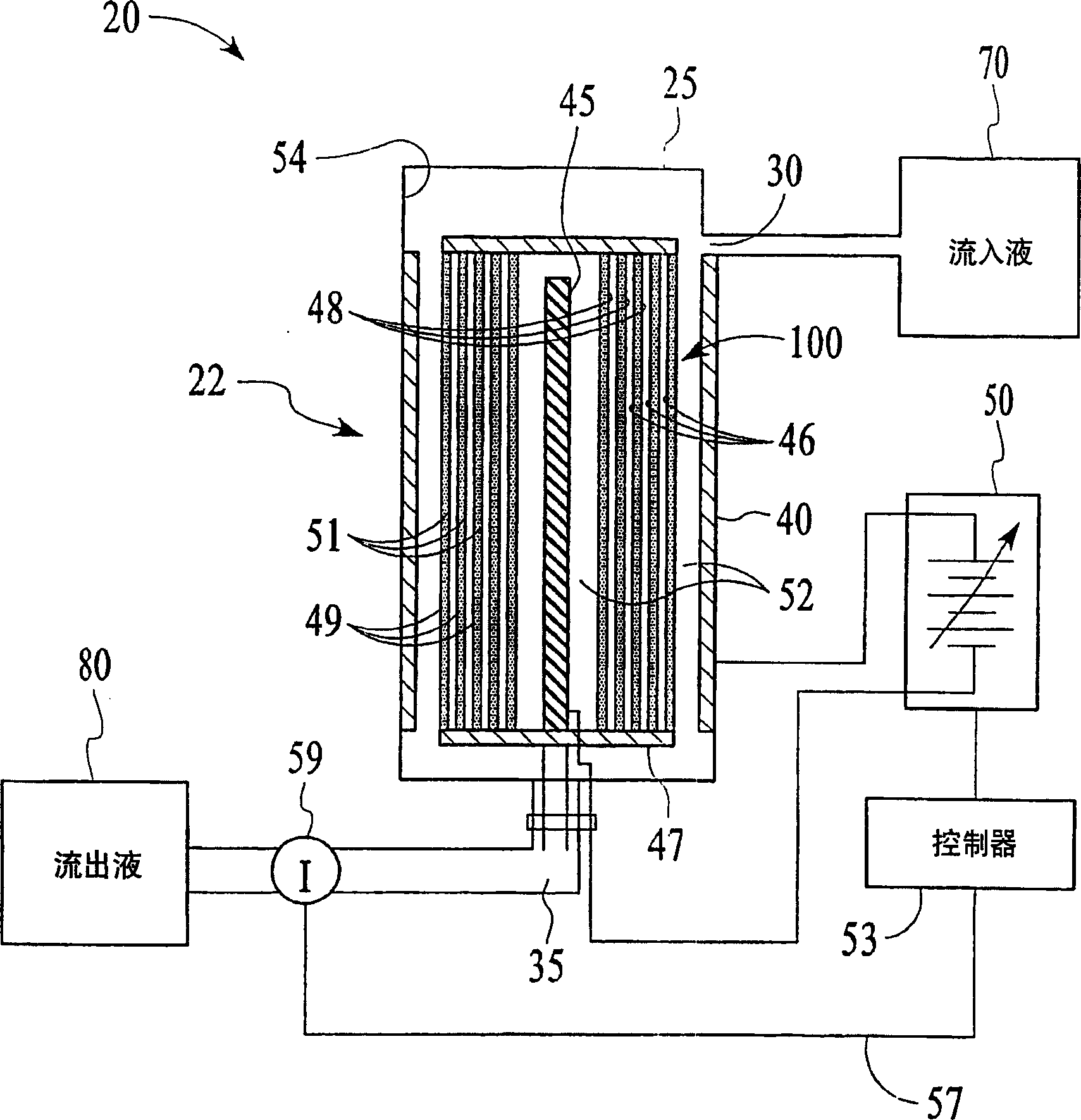
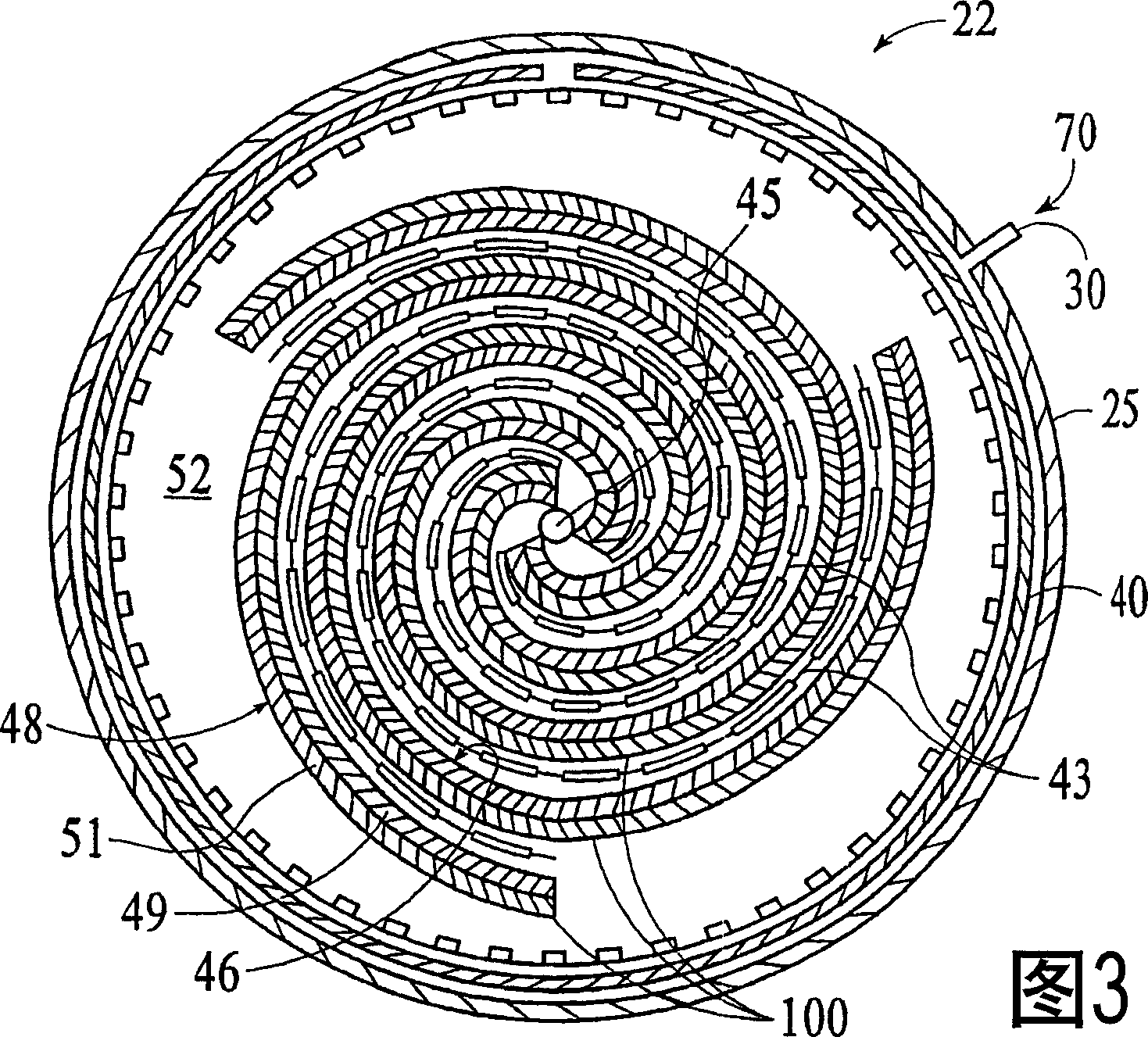
[0077] This embodiment typically measures the loss of ion removal efficiency of the hydrolyzed ion exchange membrane 100 in one ion removal cycle. The TDS content reduction test was conducted using a cell 22 including a helically wound hydrolysis membrane 100 in a cylindrical casing 25 . A constant voltage power supply with a current limit of 1A is used, so no feedback circuit is used. Cell 22 includes 30 layers of membrane 100 between a center electrode and an outer electrode. Membrane 100 has cation exchange layer 51 facing outward and anion exchange layer 49 facing inward. Initially, cell 22 was regenerated by a 20-minute ion exclusion step at 160 ml / min and 300 V using a feed water flow of ~50 μS / cm to prepare it for a deionization or ion removal step. During this regeneration step, the current to the electrodes 40 , 45 is maintained at 0.5-1.0 amps, and the influent 70 flows from the inside to the outside of the spiral membrane 100 ...
Embodiment 2
[0079] Example 2: Variable Voltage
[0080] In this embodiment, the device 20 includes the battery 22 of Embodiment 1 with an ion sensor 59 that is connected to a controller 53 (which includes a microprocessor to interpret the ion concentration signal and to control the output voltage of the variable voltage power supply 50). ) connected conductivity sensor. The target ionic conductivity for this test is 125 μS / cm. A preliminary test determines the output voltage for different deviations of the 80 ionic conductivity of the effluent from the target conductivity. The results are shown in Figure 11 variable voltage curve. When the measured conductivity exceeds the target of 125 μS / cm, the controller 53 increases the voltage of the cell 22 and the conductivity of the effluent 80 decreases shortly thereafter. The slow rise and fall changes of effluent 80 are a result of the finite response time of the system. This depends primarily on the flow rate and void volume of the cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com