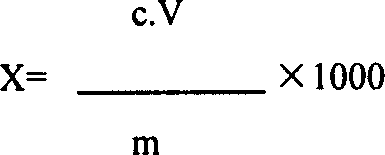Production of epsilon-caprolactam
A kind of technology of caprolactam and cyclohexanone oxime, which is applied in the field of preparation of ε-caprolactam
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
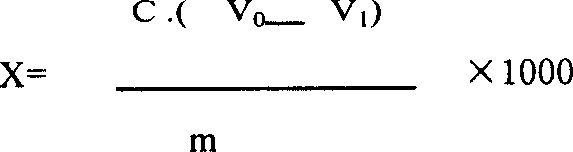
[0053] In a fixed-bed reactor equipped with RBS-1 zeolite catalyst, the reaction at 380 °C after heating the mixture of cyclohexanone oxime, methanol and ethanol (2:1), water (1:2:0.02 by weight) and nitrogen The Beckmann rearrangement reaction is carried out at high temperature, and the residence time is 8Sec. The reaction gas was cooled, part of the solvent was removed, and a reaction product containing 18 wt% of alcohol, 4 wt% of other impurities and 78 wt% of ε-caprolactam was obtained.
[0054] 500g of the above reaction product was stirred and crystallized at 20°C, and then centrifuged to obtain 308g of crude ε-caprolactam with a purity of 99.5wt%, and 192g of mother liquor. The mother liquor was distilled to obtain 78g of crude ε-caprolactam with a content of 99.3wt%. , The yield of ε-caprolactam is 98.1%. Two kinds of crude ε-caprolactam are mixed with 1500g benzene to form a benzene solution containing 20wt% ε-caprolactam, which is extracted 8 times with 1280g desalte...
Embodiment 2
[0057] According to the method of Example 1, take 500g of the reaction product obtained by stirring and crystallizing at 30°C, and then perform centrifugation to obtain 268g of crude ε-caprolactam with a purity of 99.75wt%, and obtain 232g of mother liquor. After removing 41g of the alcohol solvent in the mother liquor , the remaining 191g was stirred and crystallized at 30°C. After centrifugation, 83g of crude ε-caprolactam with a content of 99.5wt% was obtained. After separation, the mother liquor was distilled to obtain 36g of ε-caprolactam, with a yield of 99.1%. The crude ε-caprolactam obtained above was miscible with 900g benzene to form a benzene solution containing 30wt% ε-caprolactam, extracted 8 times with 580g desalted water to form an aqueous solution containing 40wt% ε-caprolactam, and passed through 50g adsorption at a speed of 5g / min. resin bed.
[0058] Take 600g containing 40wt% ε-caprolactam aqueous solution, with 30h -1 The space velocity is passed through ...
Embodiment 3
[0060] According to the method of Example 1, take 500g of the reaction product obtained by stirring and crystallizing at 36°C, and then perform centrifugation to obtain 238g of crude ε-caprolactam with a purity of 99.8wt%, and obtain 262g of mother liquor. After removing 40g of the alcohol solvent in the mother liquor , the remaining 222g was stirred and crystallized at 30°C. After centrifugation, 101g of crude ε-caprolactam with a content of 99.46wt% was obtained, and the remaining mother liquor was distilled to obtain 45g of ε-caprolactam, with a yield of 98%. The crude ε-caprolactam obtained above was miscible with 580g of benzene to form a benzene solution containing 40wt%ε-caprolactam, extracted 8 times with 390g desalted water to form an aqueous solution containing 48wt%ε-caprolactam, and passed through 50g adsorption at a speed of 5g / min. resin bed.
[0061] Take 600g containing 48wt% ε-caprolactam aqueous solution, with 30h -1 The space velocity is passed through a ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com