Analysis method of mercapto amine tropine content
A technology of mercaptotropine and analysis method, which is applied in the field of instrument analysis, can solve the problems of lack of chromophoric groups, difficulty in detection by ultraviolet detector, and no content detection method reported in literature, and achieve the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
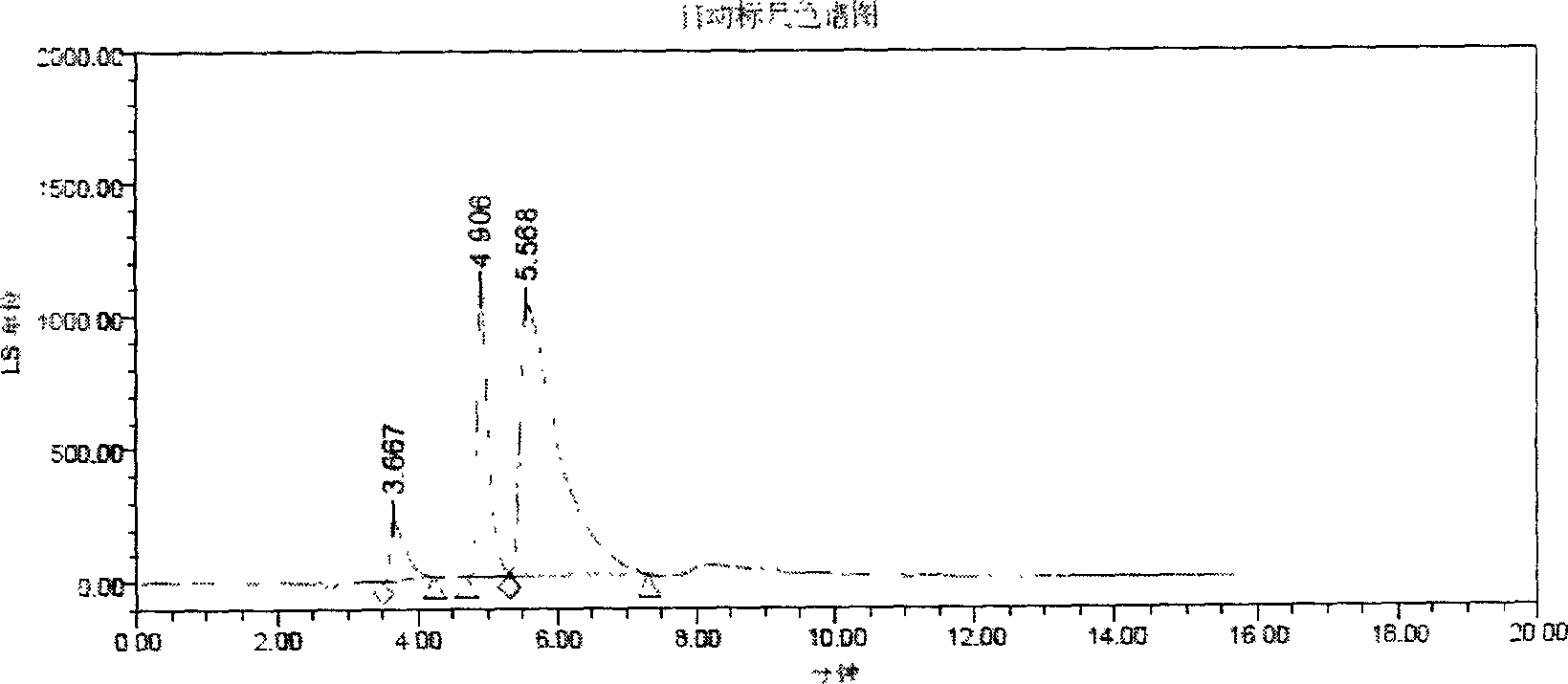
[0015] The selection of embodiment 1 mobile phase
[0016] The reaction formula of preparing thiolamine tropine is:
[0017]
[0018] Since compound 9 (2β-[N-[2-[S-(4-methoxybenzyl)thio]ethyl]-N-[[N-[2-[S-(4-methoxybenzyl) base)thio]ethyl]amino]formylmethyl]aminomethyl]-3β-(4-chlorophenyl)tropane) and compound 10 (2β-[N-[2-[S-(4- Methoxybenzyl)thio]ethyl]-N-[2-[N-[2-[S-(4-methoxybenzyl)thio]ethyl]amino]ethyl]aminomethyl ]-3β-(4-chlorophenyl)tropane) is the synthetic intermediate of TRODAT-1; therefore, the separation of TRODAT-1 and them should be investigated. The following solutions were respectively used as mobile phases, and experiments were carried out with a C8 column (Symmetry, 5 μm, 4.6×150 mm).
[0019] (A) Methanol-water (80:20)
[0020] (B) Methanol-water (60:40)
[0021] (C) Acetonitrile - water (80:20)
[0022] (D) Acetonitrile-water (60:40)
[0023] (E) methanol-water-TFA (80:20:0.1)
[0024] (F) Acetonitrile-water-TFA (80:20:0.1)
[0025] (G) methano...
Embodiment 2
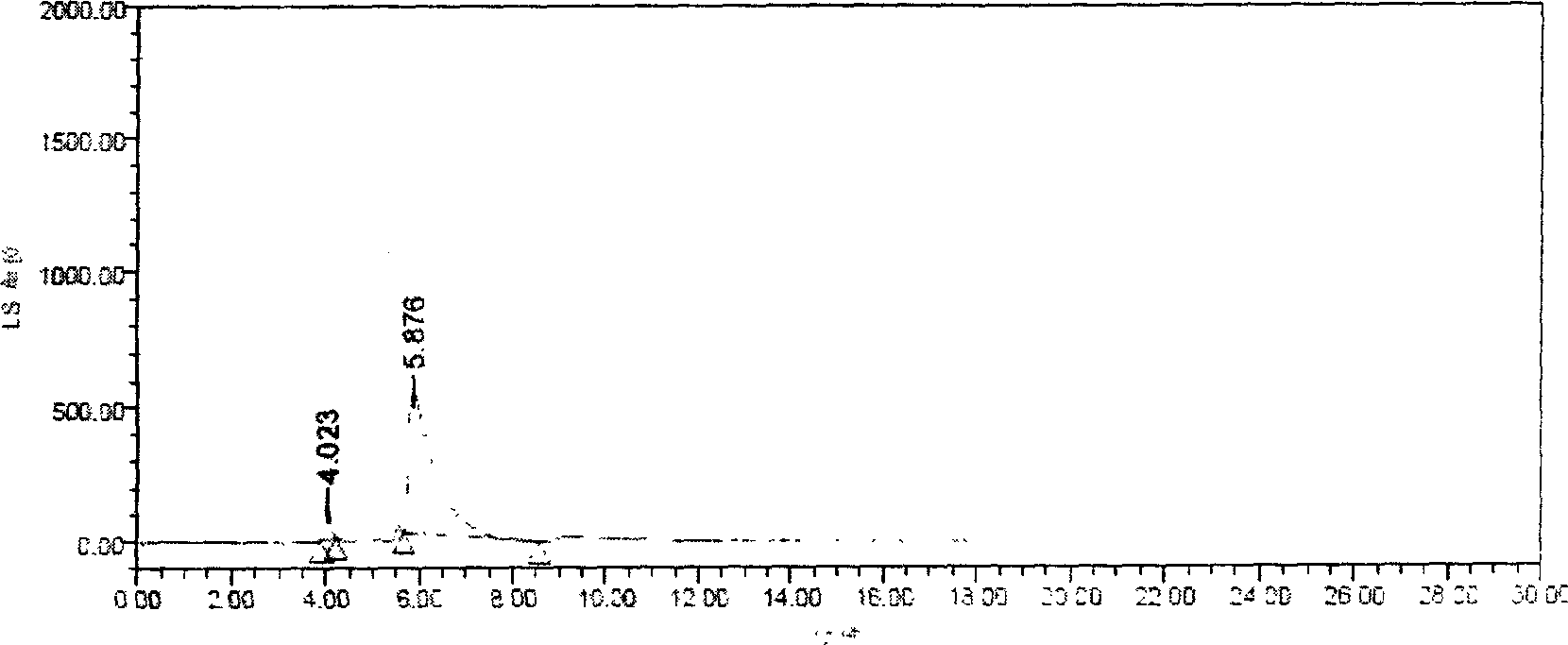
[0027] Example 2 Setting of drift tube temperature
[0028] The temperature of the drift tube was set at 40°C to 100°C for experiments, and the results showed that the chromatographic separation was better when the temperature of the drift tube was 60°C to 80°C. Therefore, the chromatographic conditions are determined as follows: Instruments: WATERS 600 high performance liquid chromatography, WATERS 2420 evaporative light scattering detector. Chromatographic column Hypersil C8 column, 5μm, 4.6×250mm, mobile phase: methanol-acetonitrile-water-TFA (80:10:10:0.1), filter and degas through a 0.45μm organic filter membrane before use, flow rate: 1.0ml / min; injection volume: 5μl; column temperature at room temperature; ELSD drift tube temperature 60℃~80℃, carrier gas (N 2 ) pressure is 35psi.
Embodiment 3
[0029] Embodiment 3 carrier gas (N 2 ) flow setting
[0030] Carrier gas (N 2 ) pressure selection 15, 25, 35, 45psi test, the chromatographic effect is better when the pressure is 35psi.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com