Reversing agent for drug-fast during treating tumor with multiple medicines
A tumor cell, multi-drug resistance technology, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of research reports and patent disclosures that have not been seen in reversing tumor multi-drug resistance by pregnane compounds , to achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
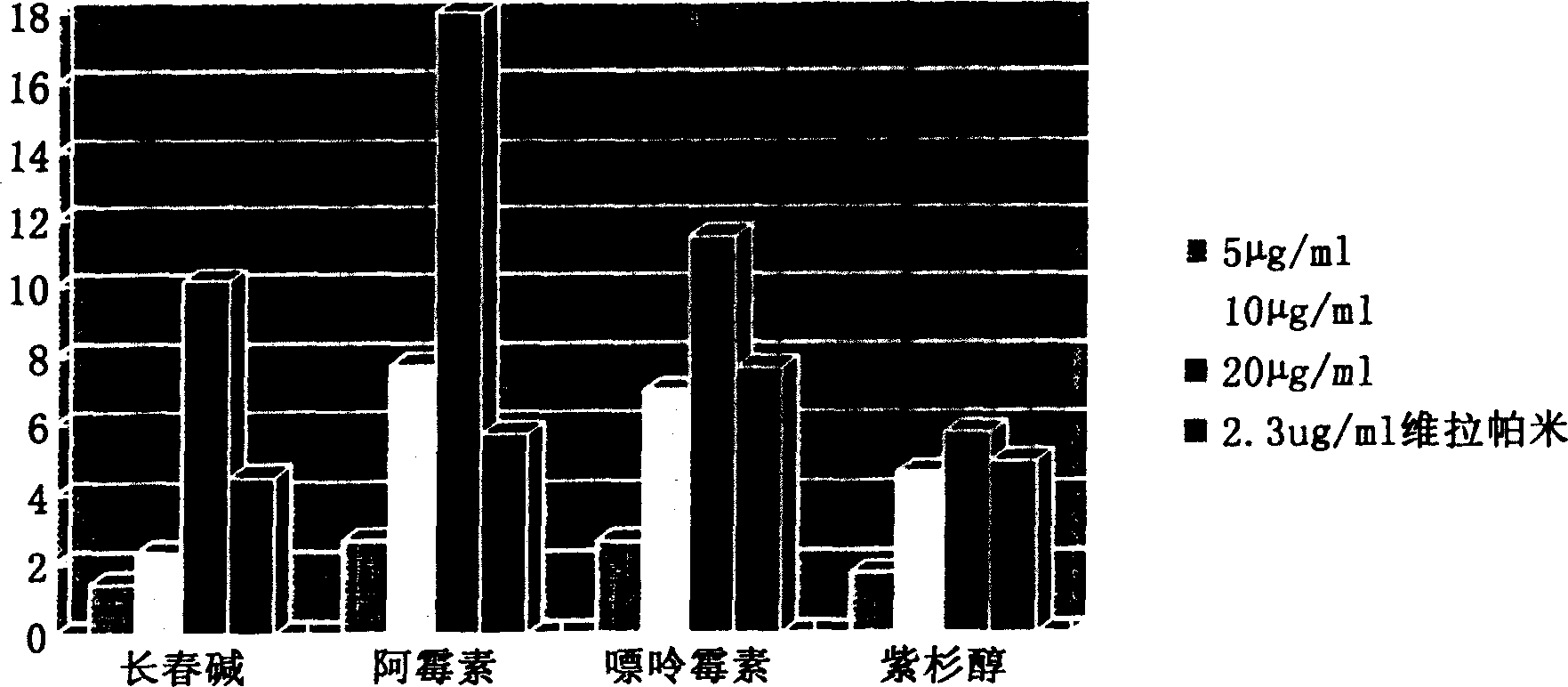
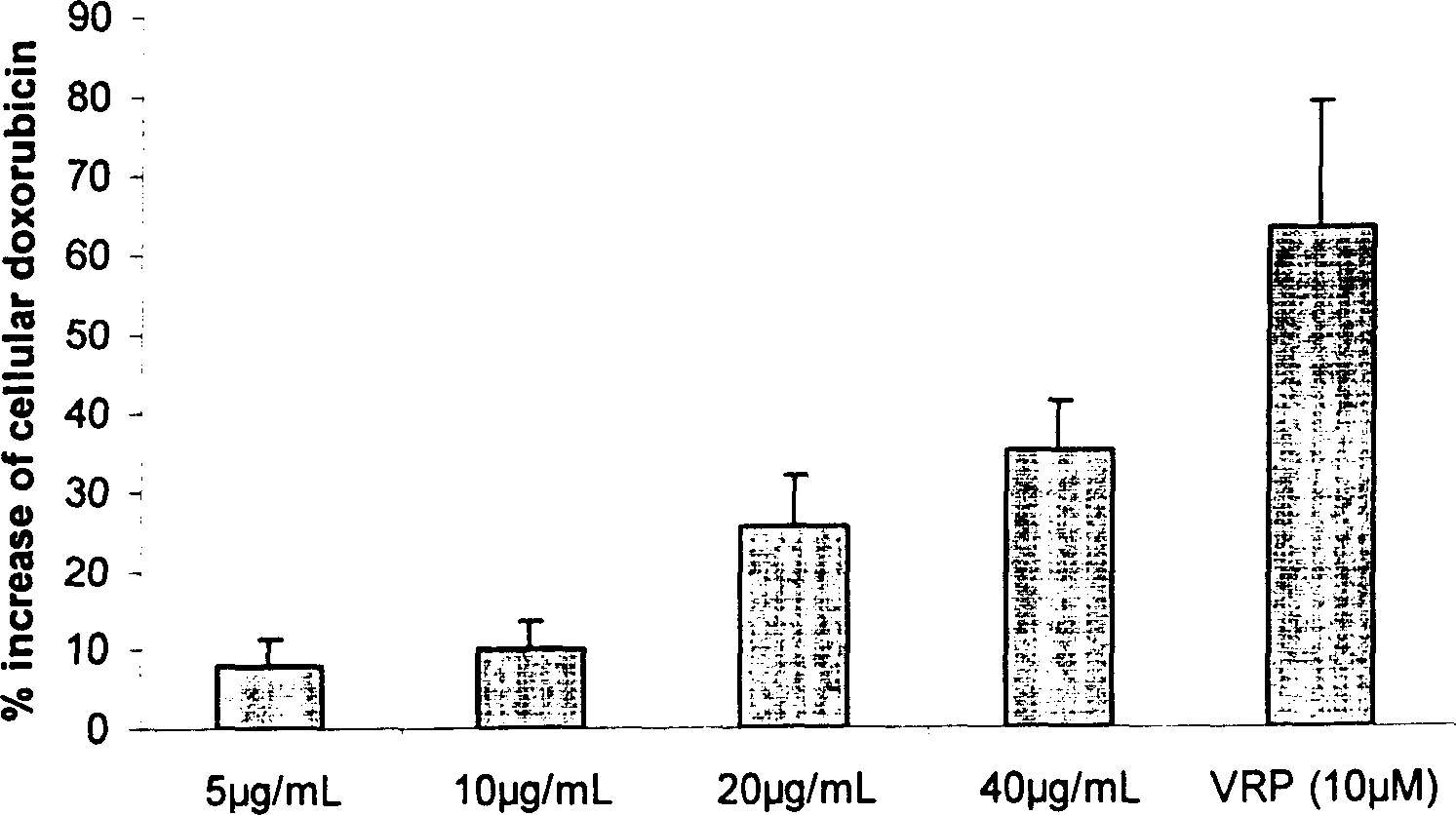
Image
Examples
Embodiment 1
[0039] Embodiment 1 extraction and separation
[0040] 5.0 kg of Tongguangsan coarse powder was added with 80% ethanol by volume, heated and refluxed for extraction 3 times, each time for 2 hours. The extract was collected, ethanol was evaporated under reduced pressure, water was added to form a suspension, petroleum ether was added to degrease, the liquid was separated, the aqueous phase was extracted with ethyl acetate, the organic phase was separated and concentrated under reduced pressure to obtain 146.5 g of ethyl acetate extract. Take the ethyl acetate fraction (80 g), add chloroform-methanol mixture to dissolve, perform silica gel column (particle size 200-300 mesh) chromatography, and use chloroform:methanol (100:0-0:100, volume ratio) gradient elution, The eluate was collected, the separation results were detected by TLC, similar fractions were combined, and repeated silica gel column chromatography, C-18 alkylated silica gel reverse phase column chromatography and pr...
Embodiment 2
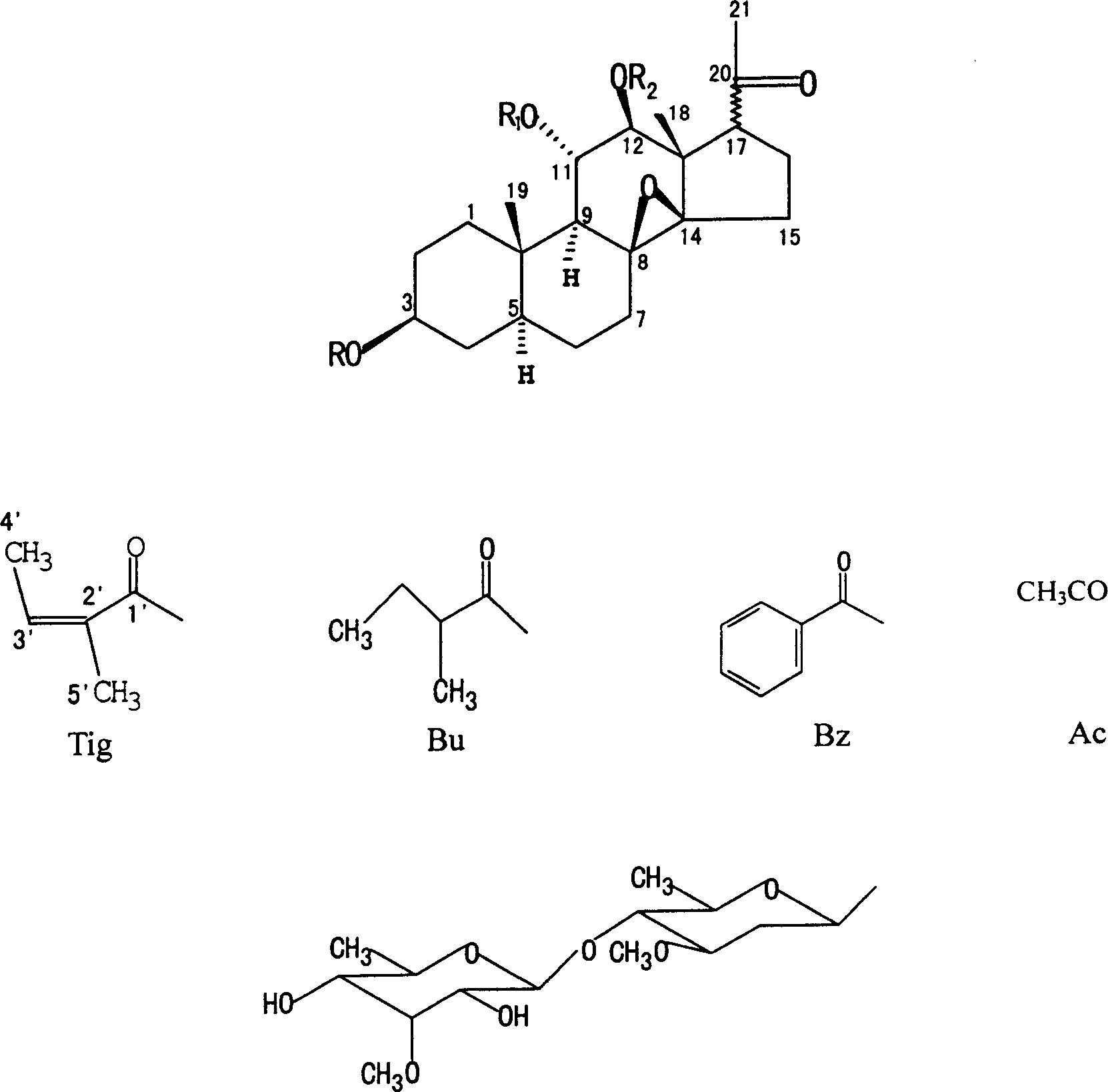
[0042] The structure identification of embodiment 2 compound MT-1 (structural formula sees accompanying drawing, the same below)
[0043] MT-1: 17βH, R = Pachbiosyl, R 1 =Tig,R 2 = Ac
[0044] Compound MT-1, C 42 h 64 o 14 . White amorphous powder, easily soluble in chloroform, methanol, acetone and other organic solvents, insoluble in water. [α] D 25 -26.3 (c0.076, CH 3 OH), the molecular formula is C 42 h 64 o 14 , ESI-MS: m / z 815.2 ([M+Na]+), 1HNMR (400MHz, CDCl 3 , δin ppm) and 13C NMR data are shown in Table 1 and Table 3. The Liebermann reaction and Keller-Kiliani reaction of MT-1 were both positive, indicating that it may be a steroidal glycoside containing 2-deoxysugar. 13 The C NMR (DEPT) spectrum shows that it contains 42 carbon atoms, including 10 methyl groups, 8 methylene groups, 16 methine groups and 8 quaternary carbons. Combined with the analysis of the biogenic characteristics of the plant containing 21-carbon steroidal ester glycosides, the che...
Embodiment 3
[0046] The structural identification of embodiment 3 compound MT-9, MT-10
[0047] MT-9: 17βH, R=H, R 1 = Bu, R 2 = Ac
[0048] MT-10: 17βH, R=H, R 1 =Bz,R 2 = Ac
[0049] MT-9,C 28 h 42 o 7 . White powder, easily soluble in chloroform, methanol, acetone and other organic solvents, insoluble in water. [α] D 25 -9.6 (c 0.052, CH 3 OH), ESI-MS: m / z 513.1 ([M+Na] + ), 1H NMR (400MHz, CDCl 3 , δin ppm) and 13 See Table 1 and Table 3 for CNMR data. Compound MT-9 13 C NMR (DEPT) spectrum shows 28 carbon atoms, including all features of MT-8 aglycone moiety: C 21 Pregnant + Acetate + 2-Methyl-2-Butenoate. Including the C-20 keto group (δ C 210.8); Acetyl (δ C 170.7 / 20.5), 2-methyl-2-butenoate (Tiglic acid) (δ C 167.2, 138.0, 128.6, 14.4, 12.6); 8β, 14β epoxy (δ66.7 and δ71.8), etc. MT-9 1 In the H NMR spectrum, δ5.330 (1H, t, J=10.0Hz) and 5.013 (1H, d, J=10.0Hz) are 11α-, 12β-ester-substituted C 21 Steroidal 11β-H, 12α-H features; 2.988 (1H, d, J = 7.6Hz) for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com