Method for producing polymer compound
A technology of polymer compounds and polymer electrolytes, applied in electrochemical generators, fuel cell components, electrolyte holding devices, etc., can solve problems such as insufficient water resistance and solvent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
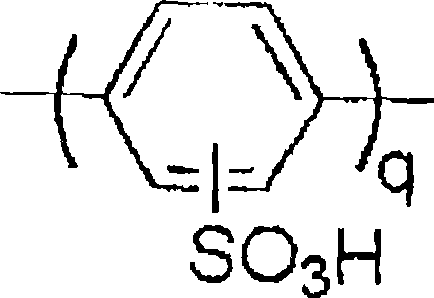
[0089] When the ambient atmosphere is Ar, add 3.84g (14.5mmol) of potassium 2,5-dichlorobenzenesulfonate, 6.23g (39.88mmol, 2.75eq) of 2,2'-dipyridine and 70mL of DMSO into the flask, and heat up under stirring To 80°C, 9.97 g (36.25 mmol, 2.5 eq) of bis(1,5-cyclooctadiene) nickel (0) was added at this temperature. Then, it was kept under stirring at this temperature for 5 hours. After standing to cool to room temperature, inject the reaction liquid into the excess methanol, filter the generated black polymer, and use 6mol / dm 3 Washed with aqueous HCl solution, the obtained polymer was dispersed at 6mol / dm 3 In the HCl aqueous solution, salts were removed by dialyzing through a dialysis membrane, and water was distilled off under reduced pressure to obtain 1.19 g of poly-p-phenylenesulfonic acid. The molecular weights of the obtained substances were as follows, and were the same as those in the sample when incubated for 3 hours.
[0090]
[0091] Mn=2.4×10 5 q+r150...
Embodiment 2
[0094] In Example 1, except that when the temperature was raised to 45°C, bis(1,5-cyclooctadiene) nickel (0) was added at this temperature, and then the temperature was raised to 80°C in 1 hour, and kept stirring at this temperature for 5 Except for these changes, the rest were carried out according to Example 1 to obtain 1.03 g of poly-p-phenylenesulfonic acid. The molecular weights of the obtained substances were as follows, and were the same as those in the sample when incubated for 3 hours.
[0095]
[0096] Mn=7.0×10 4 q+r400(r=0)
[0097] The obtained polymer compound had good film-forming properties, no cracks, good solvent resistance, and no weight loss.
Embodiment 3
[0099] In Example 1, in addition to using 1.50 g (5.66 mmol) of potassium 2,5-dichlorobenzenesulfonate, 3.98 g (15.84 mmol) of 2,5-dichlorobenzophenone and 9.72 g of 2,2'-dipyridine (62.23mmol, 2.89eq), the temperature was raised to 60°C, and after adding 15.56g (56.57mmol, 2.63eq) of bis(1,5-cyclooctadiene) nickel (0) at this temperature, the temperature was raised to 80°C in 1 hour. ℃, kept stirring at this temperature for 6 hours except for these changes, the others were reacted according to Example 1. After cooling, add the reaction solution to 6mol / dm 3 In aqueous HCl solution, filter the resulting precipitate, and use 6mol / dm 3 After washing several times with aqueous HCl solution, it was dispersed in boiling water, stirred for 2 hours, filtered, and washed twice to obtain 3.70 g of poly-p-phenylenesulfonic acid. The molecular weights of the obtained substances were as follows, and were the same as those of the samples at the time of incubation for 3 hours.
[0100] M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com