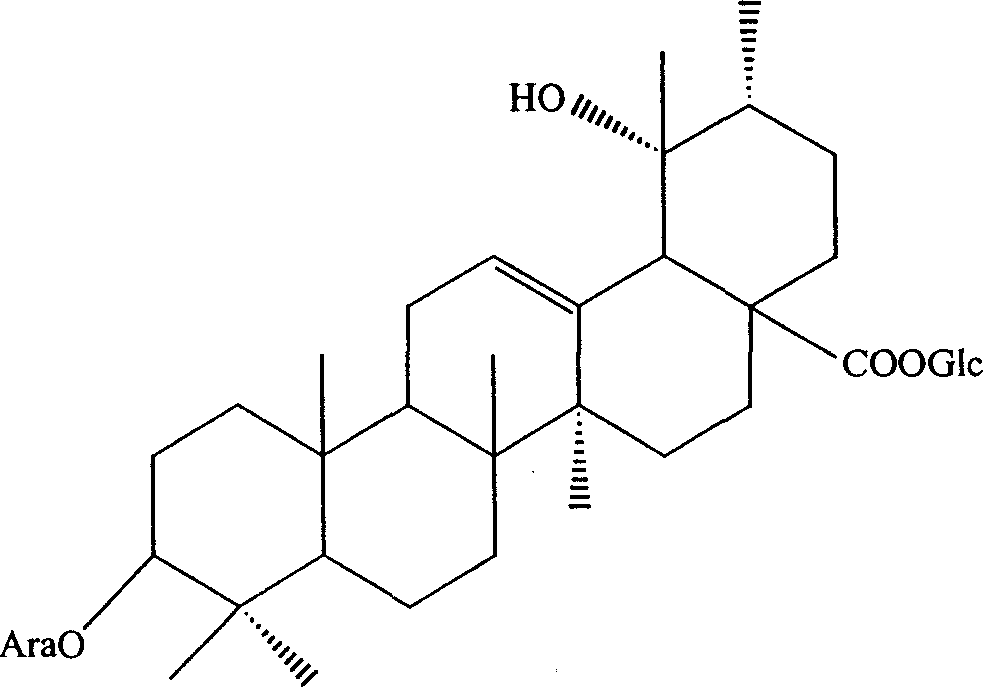
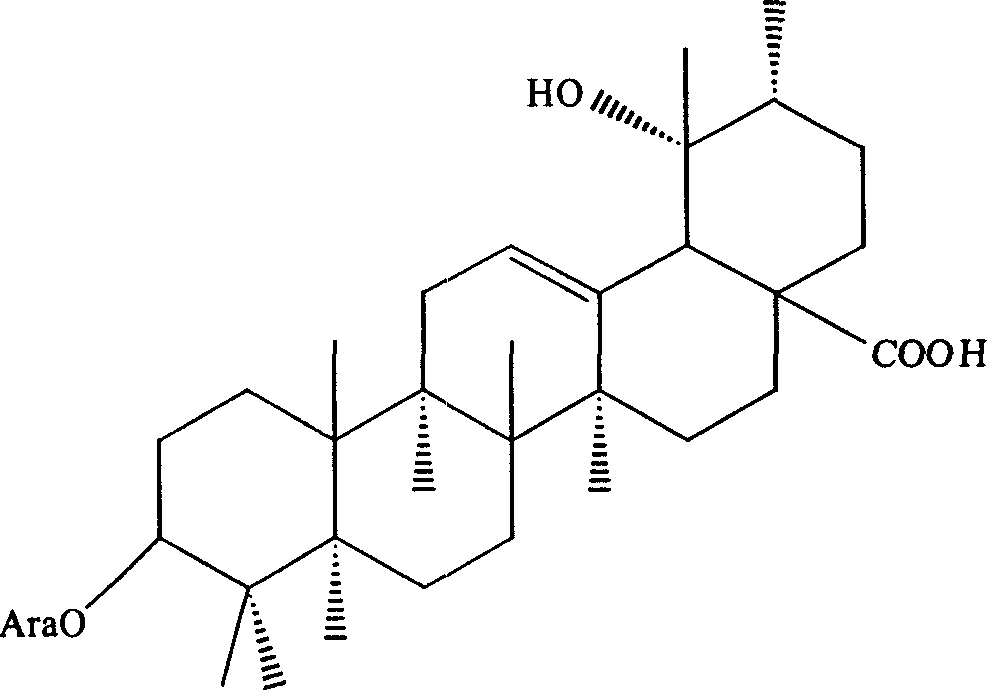
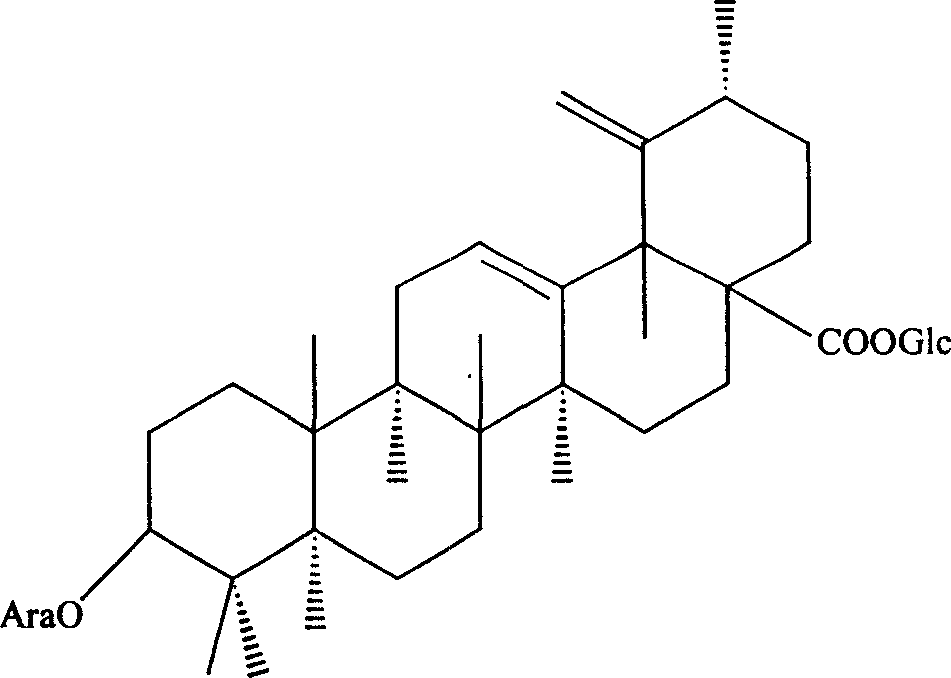
New use of radix sanguisorbae total saponin extract
A technology of Burnet saponins and total saponins is applied in the field of preparation of drug preparations for treating or preventing human leukopenia by the total saponins extract of Burnet, and achieves the effects of good leukocyte increasing effect, convenient use and remarkable curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of Burnet Burnet total saponins extract
[0035] Burnet 1000g, crushed, add 8L of 70% ethanol solution, heat and reflux for extraction for 1.5 hours, add 6L of 70% ethanol solution to the filter residue, heat and reflux for extraction for 1 hour, and filter. Combine the two filtrates, concentrate to a certain concentration, and set aside for later use. Take the polyamide column, absorb the above extract with a polyamide column, then elute the column with ethanol with a concentration of 70%, collect the eluent, recover the ethanol from the eluent under reduced pressure to obtain an extract, add water to heat to dissolve, and then The polyamide column was repeatedly adsorbed once, and finally the extract was obtained, which was vacuum-dried into a dry powder to obtain 24 g of total saponins extract of Burnet burnet, the total saponin content of which was 55% of the total weight.
Embodiment 2
[0036] Embodiment 2: Burnet Burnet total saponins extract to 60 Protective effect of Co-γ-induced bone marrow injury in mice
[0037] 60 Kunming mice, half male and half female, are divided into 6 groups: normal control group, model control group, high, middle and low dose groups of total saponins extract from Burnet burnet (total saponin extract from Burnet burnet obtained in Example 1) , batyl alcohol group. The above 6 groups of animals, except the normal control group animals, the other 5 groups of animals were treated with 1 day before administration. 60 Co-γ ray was irradiated once, with a dose of 4Gy. After 24 hours, they were randomly divided into 5 groups. Except for the normal control group and the model control group, 0.4ml of distilled water was administered intragastrically, and the rest of the groups were intragastrically administered with different doses. 1 time, for 7 consecutive days. 24 hours after the last administration, the mouse eyeball was pulled out ...
Embodiment 3
[0043] Example 3: Therapeutic effect of total saponins extract from Burnet burnet on leukopenia in mice induced by cyclophosphamide
[0044] 67 Kunming mice, half male and half male, are randomly divided into 6 groups, i.e. normal control group, cyclophosphamide model control group (Cy group), Cy+ Burnet burnet total saponins extract (for embodiment 1 gained Burnet burnet total saponins extract) high, medium and low dose groups, Cy+bakelyl alcohol group. Except for the normal control group, mice in other groups were intraperitoneally injected with cyclophosphamide (Cy) 150 mg / kg to form a leukopenia model. On the day of Cy injection, the normal group and the Cy model control group were given distilled water at 20ml / kg respectively. The rest of the groups were given different drugs according to different doses, once a day, orally, for 10 consecutive days. 24 hours after the last administration, the eyeballs were taken to take blood, and the total number of white blood cells i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com