Medicine composition containing insulin sensibilizer and B-family vatamines
A technology of insulin sensitizer and B vitamins, which is applied in the directions of drug combination, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of the prevention of complications and the unsatisfactory overall treatment effect of diabetic patients. , to achieve the effect of reducing medical costs, convenient medication, and improving patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
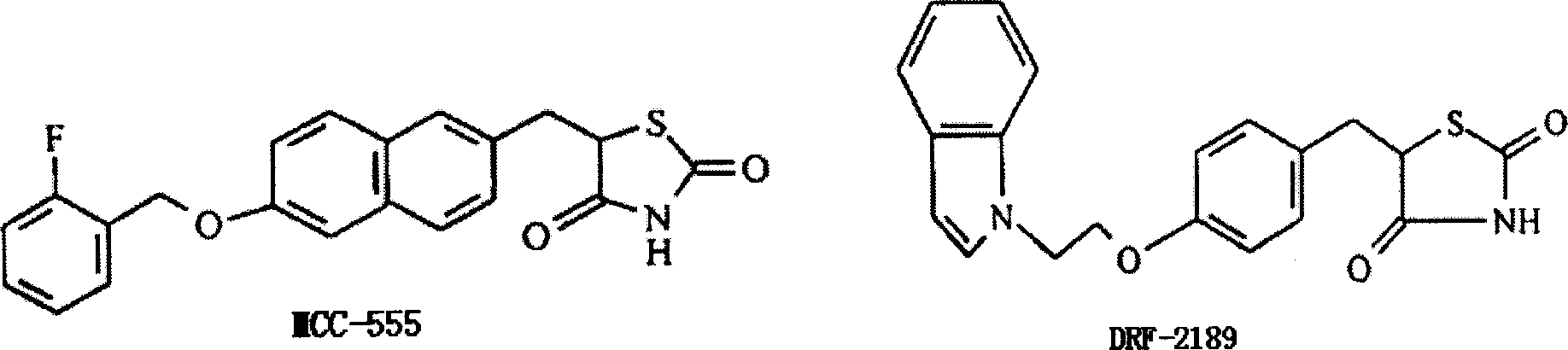
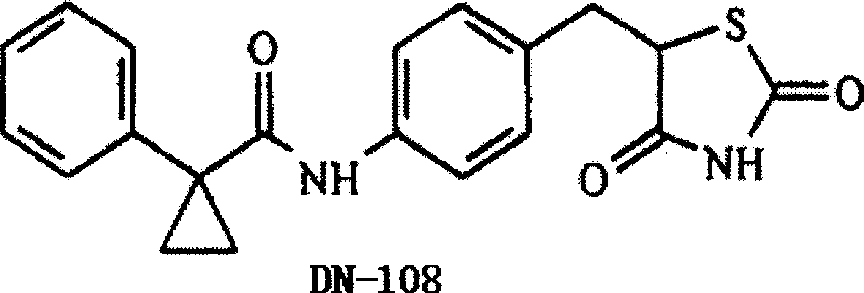
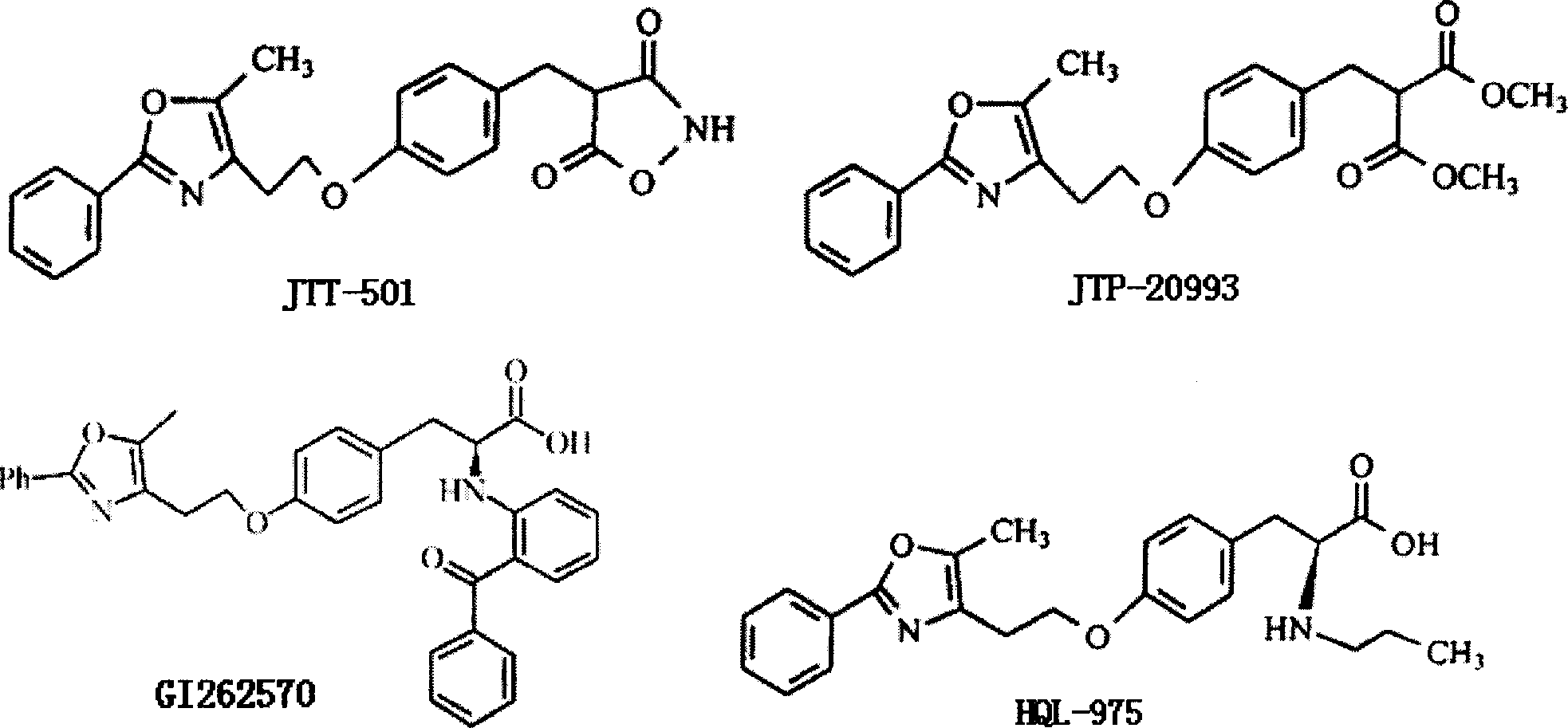
Image
Examples
Embodiment 1
[0035] Example 1. The preventive and therapeutic effects of rosiglitazone maleate and folic acid in combination in rats with diabetic nephropathy.
[0036] 100 Wistar male rats, 15 were taken as normal control group, and the remaining 85 rats were intraperitoneally (ip) injected with complete Freund’s adjuvant (CFA) and small dose of streptozotocin (STZ 30mg / kg), once a week , For 2 consecutive weeks, copy the diabetes model, and end the model building in the 4th week. The model was successfully selected (fasting blood glucose between 16.8 and 25mmol / L) rats were randomly divided into model group, rosiglitazone group (0.4mg / kg), folic acid group (0.08mg / kg), rosiglitazone + Folic acid group (0.4mg / kg+0.08mg / kg), 15 rats in each group, and a normal control group. The model group and the normal group were given equal volume of normal saline, and both were given intragastrically once in the morning for 8 consecutive weeks. Urine was collected for 24 hours, and urine microalbumin (MAL...
Embodiment 2
[0046] Example 2. Preventive and therapeutic effects of pioglitazone hydrochloride and folic acid in combination on diabetic nephropathy rats.
[0047] Of 120 Wistar male rats, 15 were taken as the normal control group, and the remaining 105 were ip CFA and low-dose STZ 25mg / kg, once a week for 2 consecutive weeks, to replicate the diabetic model, and the model was completed in the fourth week. The model was successfully selected (fasting blood glucose value ≥ 11.1mmol / L) rats were randomly divided into model group, pioglitazone group (3mg / kg), folic acid group (0.5mg / kg), pioglitazone + folic acid group (3mg / kg+0.04mg) / kg), 15 rats in each group of pioglitazone + folic acid (3mg / kg+0.5mg / kg), and a normal control group. The model group and the normal group were given equal volume of normal saline for 8 weeks. Urine was collected for 24 hours, and urine MALB and α1-MG were measured. Orbital blood was collected to measure blood glucose, serum ET, NO levels, SOD and MDA levels.
[...
Embodiment 3
[0057] Example 3. Preventive and therapeutic effects of pioglitazone hydrochloride combined with vitamin B12 on rats with diabetic nephropathy.
[0058] There are 120 Wistar male rats, and 15 of them are taken as normal control group. The experimental method is the same as that in Example 2. The model was selected successfully (fasting blood glucose value ≥ 11.1mmol / L) rats were randomly divided into model group, pioglitazone group (3mg / kg), vitamin B12 group (0.2mg / kg), pioglitazone + vitamin B12 group (3mg / kg+ 0.025mg / kg), pioglitazone+vitamin B12 group (3mg / kg+0.2mg / kg) 15 rats in each group, and a normal control group. The model group and the normal group were given equal volume of normal saline for 8 weeks. Urine was collected for 24 hours, and urine MALB and urine α1-MG were measured. Orbital blood was collected to measure blood glucose, serum ET, NO, SOD and MDA levels.
[0059] Results: Compared with the normal group, the rats in the model group showed increased blood suga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com