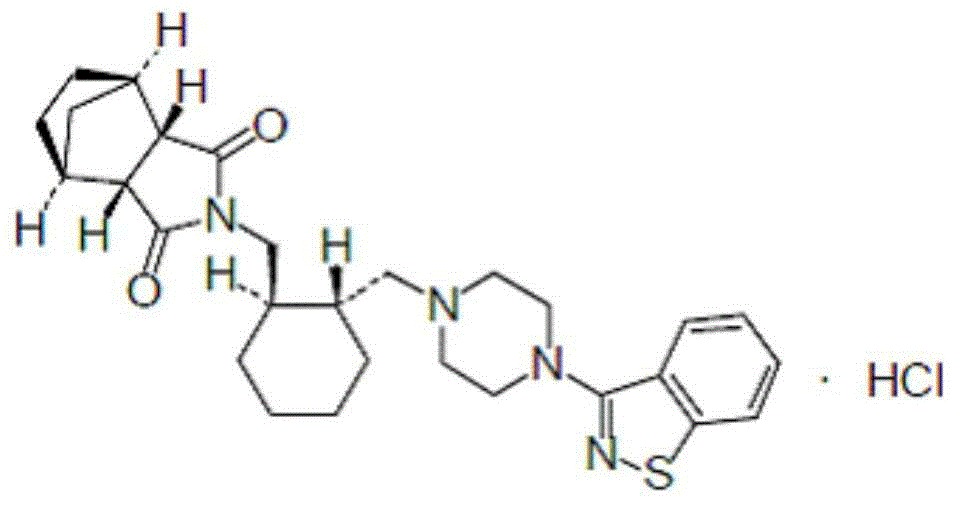
Lurasidone hydrochloride orally disintegrating tablet and preparation method thereof
A technology of lurasidone hydrochloride and orally disintegrating tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of complex operation process and lack of rapid oral disintegration , Dissolution decline and other issues, to achieve the effect of simple operation of the production process, increase of bioavailability, and improvement of dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
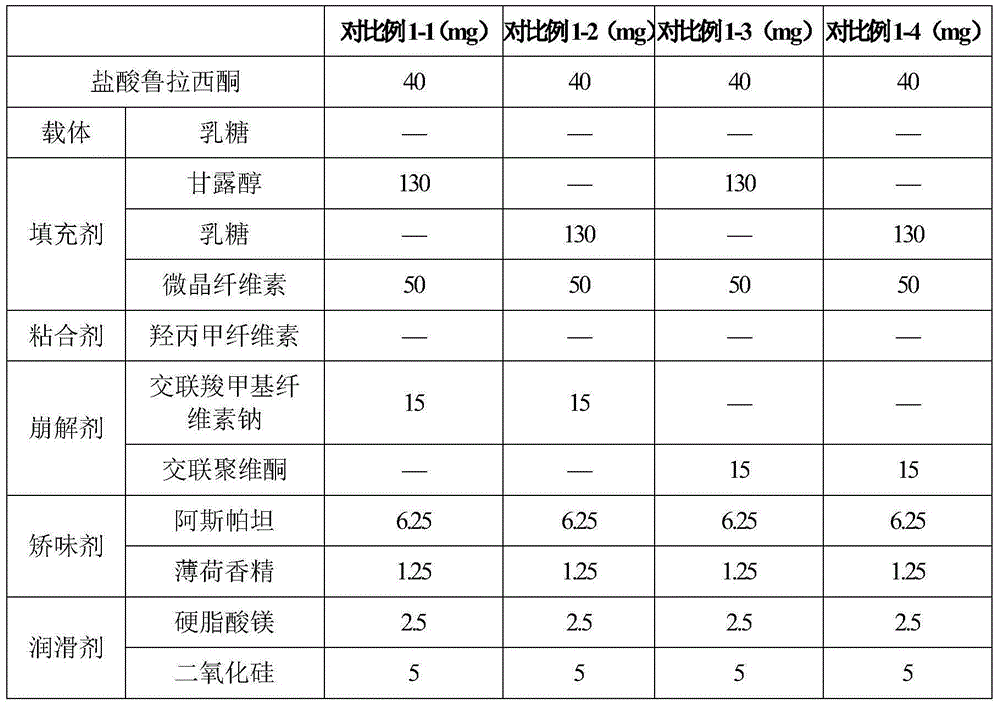
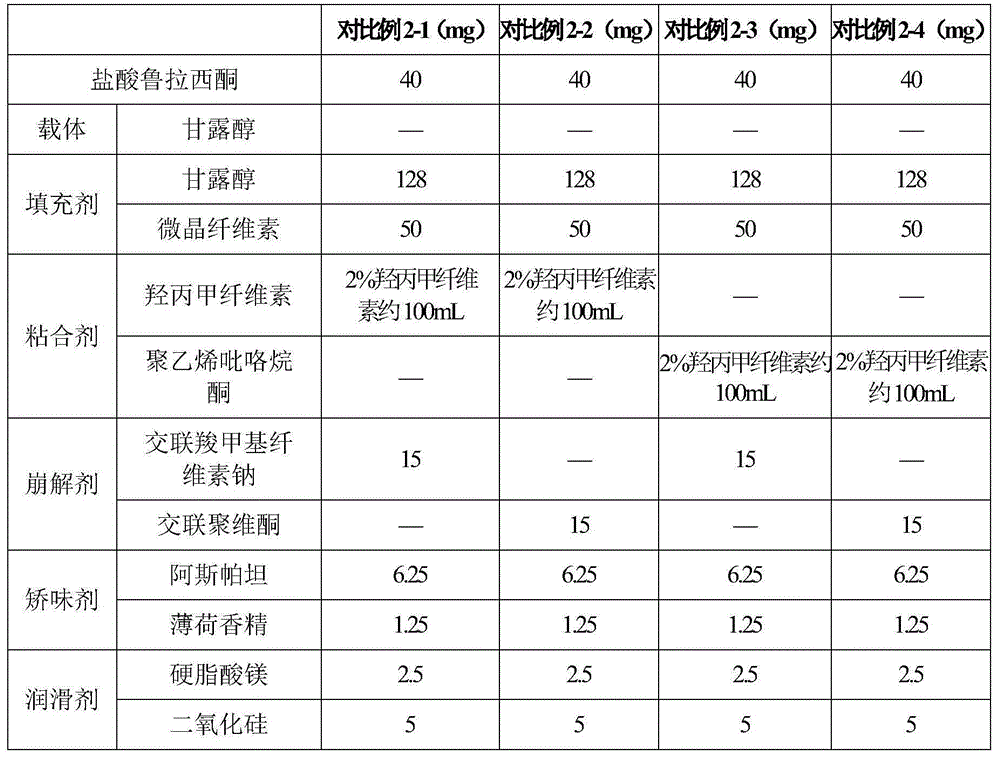
[0107] Prescription: lurasidone hydrochloride 10mg lactose (carrier) 20mg polyvinylpyrrolidone 2mg lactose 143mg microcrystalline cellulose 50mg croscarmellose sodium 17.5mg aspartame 4.5mg mint flavor 0.5mg magnesium stearate 2.5mg
[0108] Binder solvent: 40% ethanol solution 50mg
[0109]
Embodiment 2
[0111] Prescription: Lurasidone Hydrochloride 10mg Mannitol Starch Complex (Carrier) 60mg Polyvinylpyrrolidone 4mg Mannitol 93.5mg Microcrystalline Cellulose 50mg Carboxymethyl Starch Sodium 15mg Acesulfame K 13mg Strawberry Flavor 2mg Talc 2.5mg
[0112] Binder solvent: 40% ethanol solution 30mg
[0113]
Embodiment 3
[0115] Prescription: lurasidone hydrochloride 10mg mannitol (carrier) 105mg polyvinylpyrrolidone 6mg starch lactose complex 90mg sodium carboxymethyl starch 20mg acesulfame potassium 4mg lemon flavor 6mg talcum powder 9mg
[0116] Binder solvent: 40% ethanol solution 60mg
[0117]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com