Slow-release, controlled-release prepn. contg. chlortenozicam
A technology of lornoxicam and controlled-release preparations, which is applied in the direction of anti-inflammatory agents, non-central analgesics, and medical preparations of non-active ingredients. To avoid the peak and valley phenomenon, reduce the toxic and side effects, and achieve the effect of long-lasting curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of Lornoxicam Tablets
[0036] 1000 pieces
[0037] Lornoxicam 8g
[0038] Poloxamer 40g
[0039] Microcrystalline Cellulose 52g
[0040] Preparation process: Lornoxicam and poloxamer are made into solid dispersion by flux method, crushed through a 100-mesh sieve, added microcrystalline cellulose, mixed evenly, and compressed into tablets.
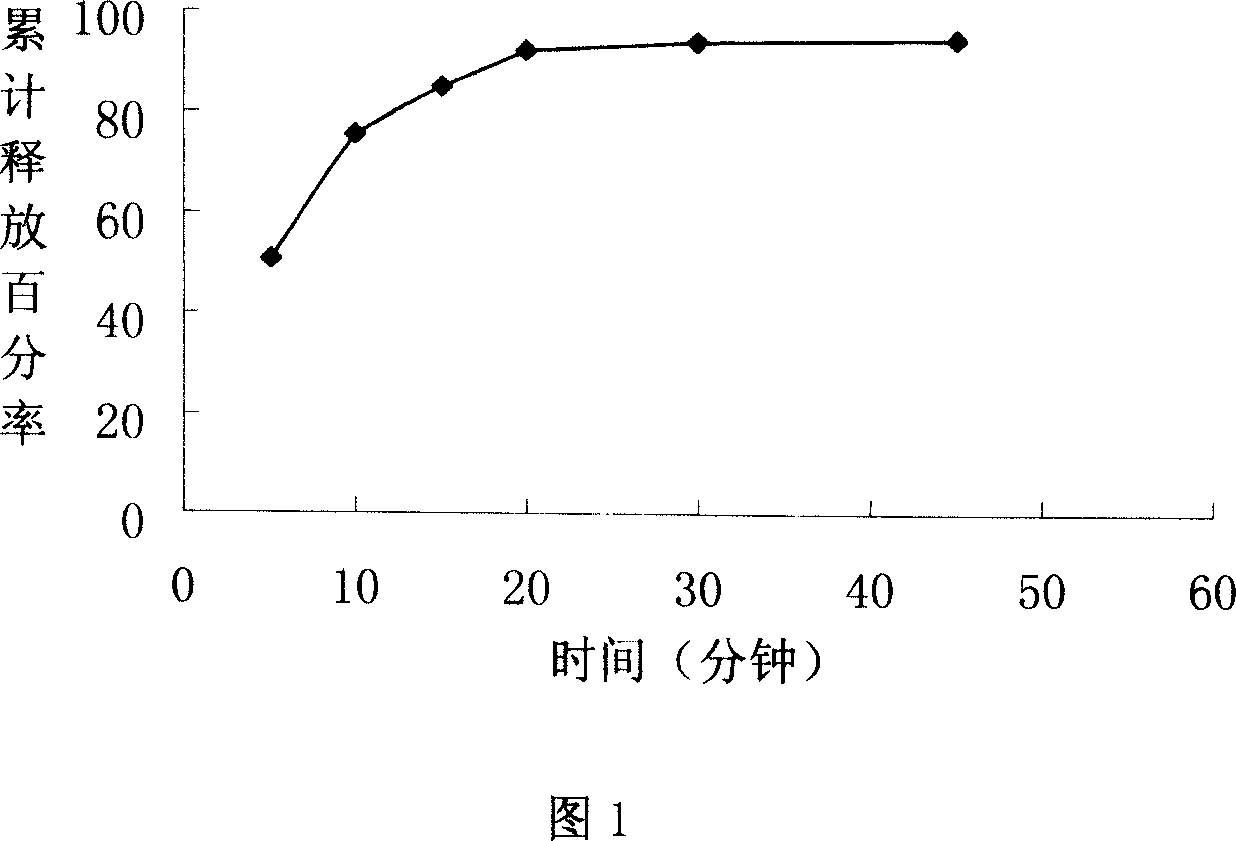
[0041] Determination of dissolution rate:
[0042] According to the dissolution determination method (Chinese Pharmacopoeia version two appendices X C third method in 2005), the phosphate buffer 250ml with PH=7.4 is the dissolution medium, and the rotating speed is 50 revolutions per minute, operated according to law, respectively in 5 minutes, 10 minutes, At 15 minutes, 20 minutes, 30 minutes, and 45 minutes, 5 ml of samples were taken, and at the same time, dissolution medium of the same temperature and volume was added, the samples were filtered, and the filtrate was taken fo...
Embodiment 2
[0043] Embodiment 2: Preparation of Lornoxicam Sustained-release Tablets
[0044] 1000 pieces
[0045] Lornoxicam 8g
[0046] Poloxamer 32g
[0047] Ethylcellulose (45cps) 10g
[0048] Microcrystalline Cellulose 44g
[0049] Preparation process: Lornoxicam and poloxamer are made into solid dispersion by solvent method, crushed through a 100-mesh sieve, mixed with ethyl cellulose and microcrystalline cellulose, and pressed into tablets. Lornoxicam: ethyl cellulose = 1: 1.25, can effectively sustained release.
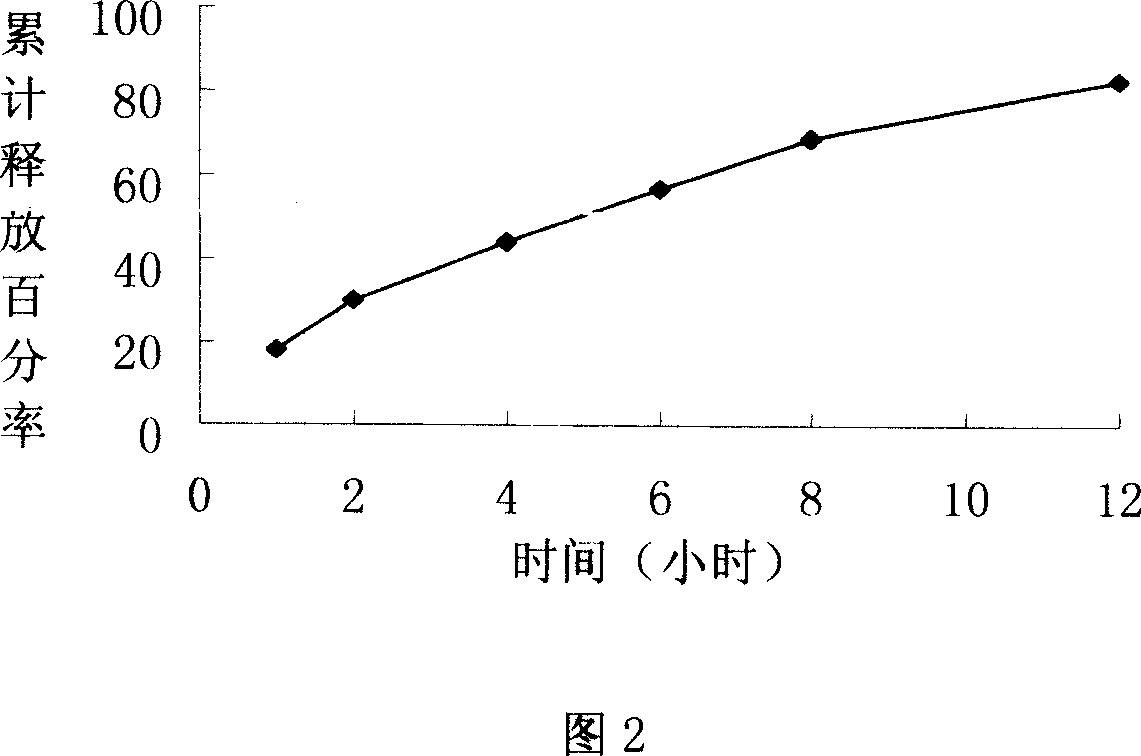
[0050] Determination of release rate of sustained-release tablets:
[0051]According to the dissolution assay method (Chinese Pharmacopoeia version two appendix X C third method in 2005), the phosphate buffer 250ml with PH=7.4 is the dissolution medium, and the rotating speed is 50 revolutions per minute, operated according to law, respectively at 1, 2, 4, Take 5ml of the solution at 6, 8, and 12 hours, add the dissolution medium at the sam...
Embodiment 3
[0052] The preparation of embodiment 3 lornoxicam sustained-release tablets
[0053] 1000 pieces
[0054] Lornoxicam 8g
[0055] Poloxamer 32g
[0056] Hypromellose K4M 20g
[0057] Microcrystalline Cellulose 44g
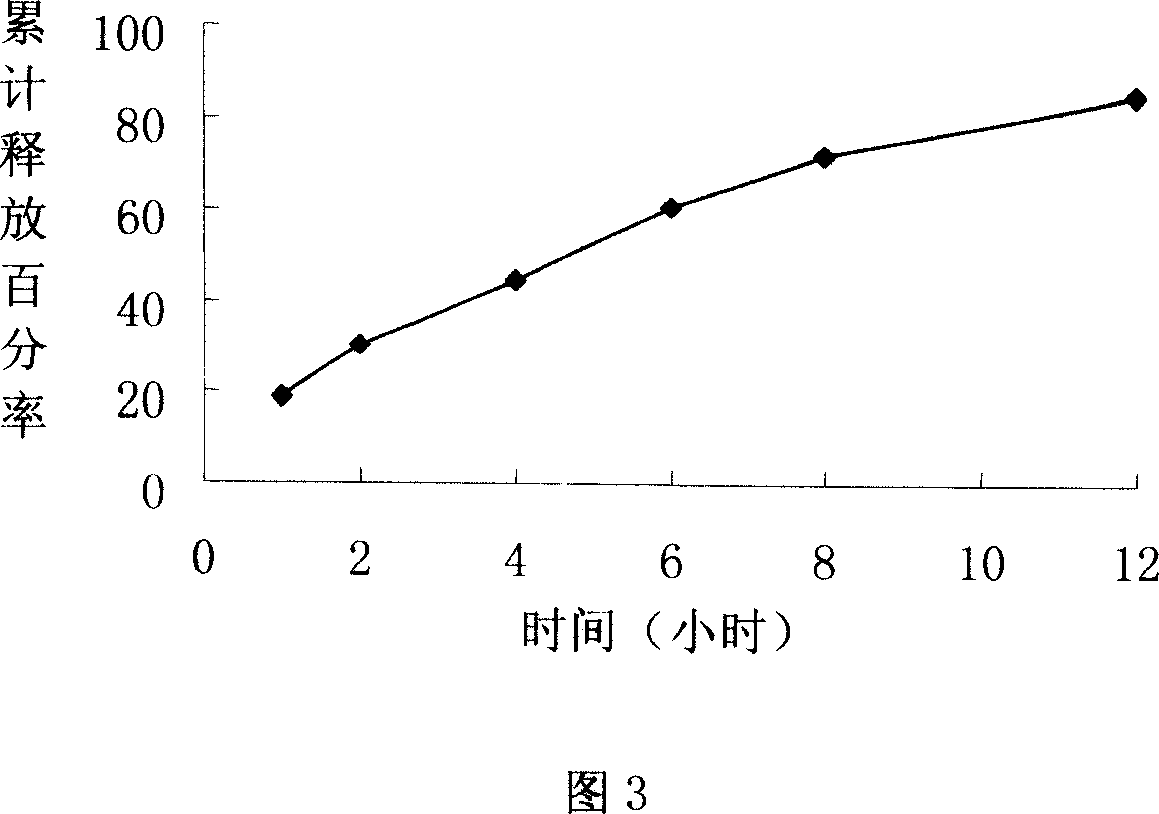
[0058] Preparation process: Lornoxicam and poloxamer are made into solid dispersion by flux method, crushed through a 100-mesh sieve, mixed with ethyl cellulose and microcrystalline cellulose, and pressed into tablets. When lornoxicam:hypromellose=1:1.25, it can be effectively sustained release.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com