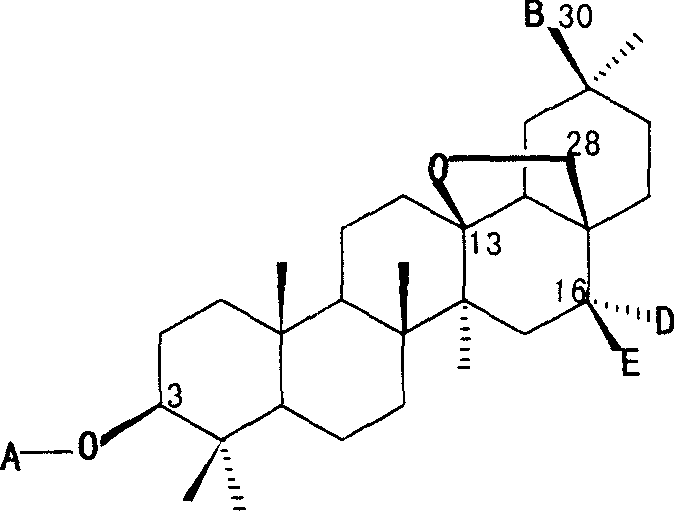
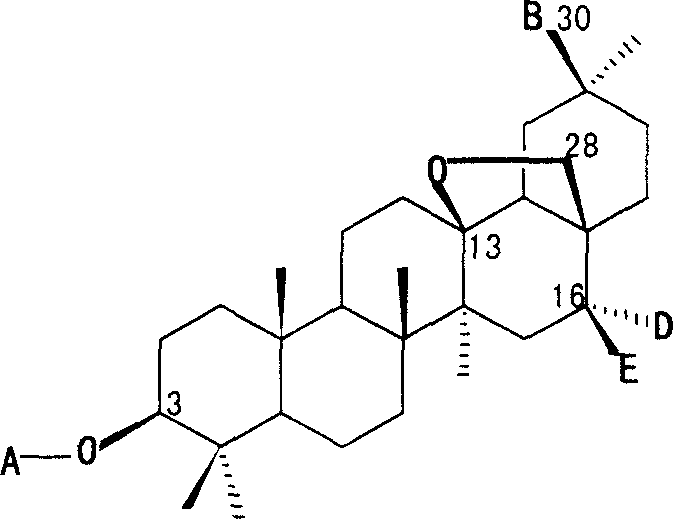
Triterpene compound for preparing drug for treating cranial glia tumour
A glioma and compound technology, applied in the field of natural medicines against glioma, can solve the problems of undiscovered high-efficiency and low-toxicity chemotherapy drugs, unsatisfactory treatment effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 extraction and separation
[0035] Crumble 1.2Kg of dried Padang Guoluhuang into powder, impregnate 3 times with 95% ethanol for 24 hours each time, combine the impregnating liquid, concentrate under reduced pressure to remove ethanol, obtain extract, suspend with warm water, and dilute the suspension with ethyl acetate The ester was extracted three times, and the extracted aqueous layer was extracted three times with n-butanol. The n-butanol part was put on a silica gel column, and the chloroform-methanol system was used as the eluent for gradient elution, and the chloroform-methanol (80:20, v / v) eluted part was subjected to repeated silica gel column chromatography to obtain compounds 1 and 2.
Embodiment 2
[0036] The structure identification of embodiment 2 compound 1
[0037] Compound 1: colorless crystals, mp 239-240°C, the molecular formula is C as determined by high-resolution mass spectrometry (HRESIMS) 53 h 86 o 22 (C 53 h 86 o 22 Tested value for Na m / z: 1097.5480, theoretical value: 1097.5503). Positive ion ESI-MSm / z: 1097.6[M+Na] + , 951.5[M+Na-rha] + , 789.4 [M+Na-rha-glucose] + ;Negative ion ESI-MSm / z: 1072.8[M-H] + , 927.0 [M-H-rha] + , 910.3 [M-H-glucose] + , 764.8 [M-H-rha-glucose] + . The 1D NMR spectrum shows that compound 1 is a plurality of glycosyl triterpene saponins, heated to reflux with 4mol / L hydrochloric acid-50% methanol solution for 4 hours, after the reaction solution was cooled and neutralized, D- Glucose (Glc), L-arabinose (Ara) and L-rhamnose (Rha). Positive ion ESI-MSm / z: 1097.6 [M+Na] + , 951.5 [M+Na-rha] + , 789.4 [M+Na-rha-glucose] + And negative ion ESI-MSm / z: 1072.8 [M-H] + , 927.0 [M-H-rha] + , 910.3 [M-H-glucose] + , 764...
Embodiment 3
[0038] The structure identification of embodiment 3 compound 2
[0039] Colorless crystals, mp 238-239°C, C 52 h 84 o 22 , Positive ion ESI-MSm / z: 1097.6[M+K] + , 1083.6[M+Na] + , 951.5[M+Na-xylose] + , 789.4 [M+Na-xylose-glucose] + ; Negative ion ESI-MS m / z: 1058.6[M-H] + , 926.5 [M-H-xylose] + , 896.5 [M-H-glucose] + , 764.4 [M-H-xylose-glucose] + . 1 H NMR (DMSO-d 6 , 400 MHz): 0.72 (3H, s, H-24), 0.79 (3H, s, H-25), 0.90 (3H, s, H-29), 0.94 (3H, s, H-23), 1.05 (3H, s, H-26), 1.16 (3H, s, H-27), 4.38 (1H, d, J=7.4, Glc inner -1″), 4.40 (1H, d, J=7.6, Glc terminal -1″″), 4.44 (1H, d, J=7.6, Ara-1′), 5.00 (1H, dd, J=7.6, Xyl-1), 9.38 (1H, s, H-30); 13 C NMR (DMSO-d 6 .100 MHz): 38.5(C-1), 25.7(C-2), 88.1(C-3), 38.8(C-4), 54.8(C-5), 17.8(C-6), 33.5(C -7), 43.6(C-8), 49.4(C-9), 36.1(C-10), 18.2(C-11), 31.8(C-12), 85.4(C-13), 41.6(C- 14), 35.6(C-15), 77.5(C-16), 42.9(C-17), 52.3(C-18), 32.4(C-19), 47.4(C-20), 29.5(C-21 ), 31.3(C-22), 27.4(C-23), 15.9(C-24), 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com