Intermediate of fathead tree medicinal materials and quality control method of injection
A quality control method and injection technology, applied in the quality control of danmu, the quality control of danmu injection, and the quality control of danmu medicinal materials, which can solve the problems of safety and effectiveness of difficult varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1. A quality control method for Danmu injection, the method contains the following assay methods,
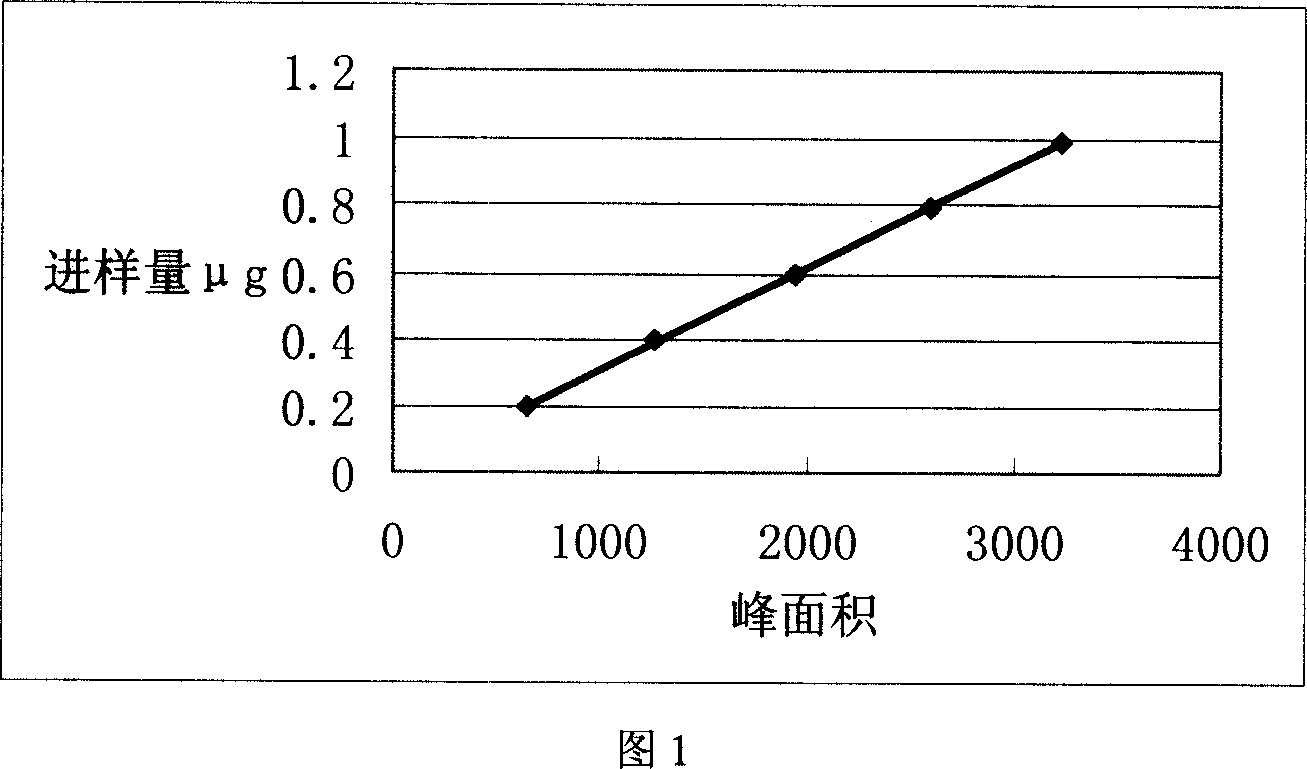
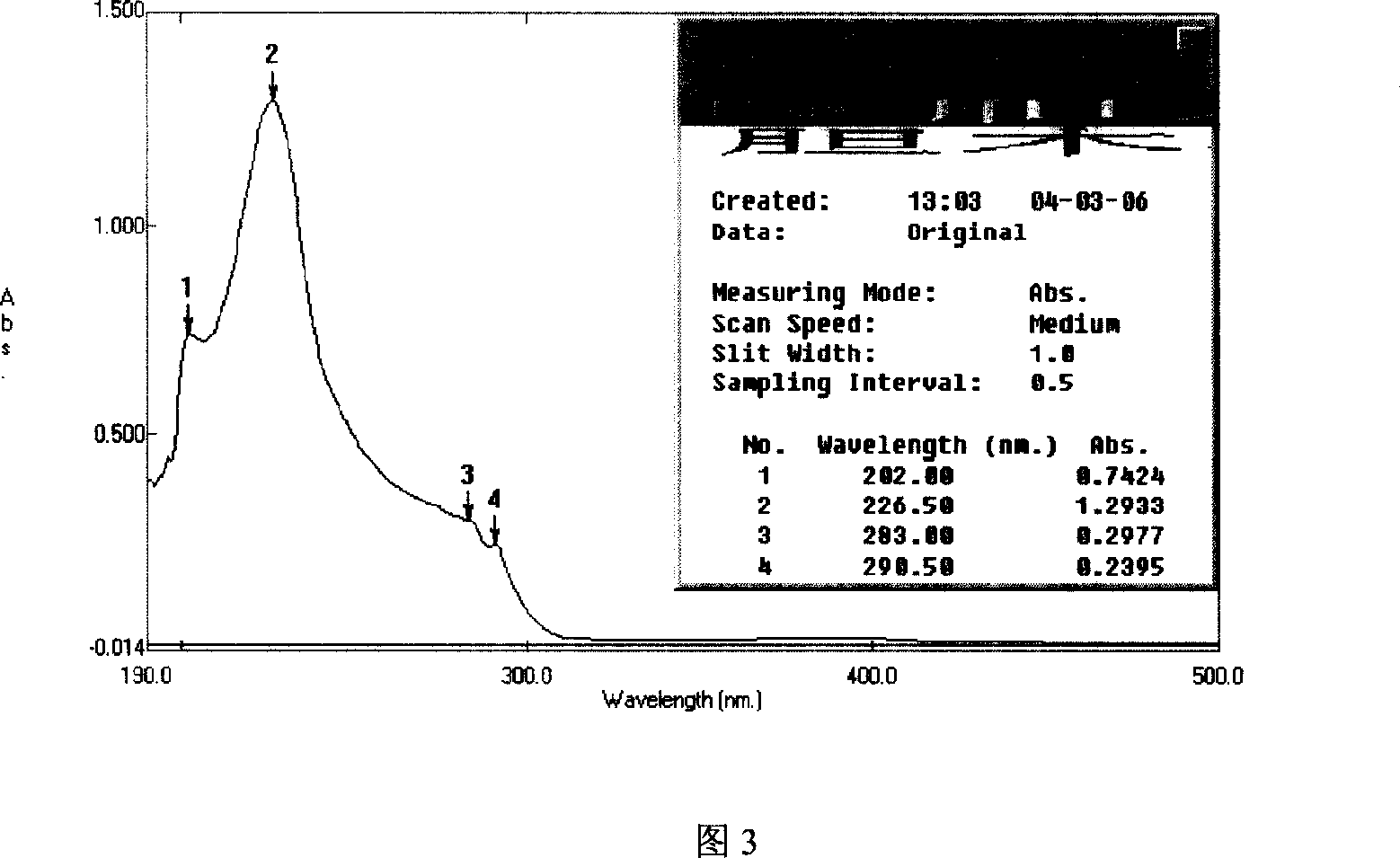
[0051] a. Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; 30:70:0.1 acetonitrile-water-phosphoric acid as mobile phase; detection wavelength is 226nm; The calculation of anthocyanin lactam should not be less than 5000;
[0052] b. Preparation of the reference substance solution: take an appropriate amount of vinblastam, accurately weigh it, add methanol to dissolve, and prepare solutions containing 100 μg / ml and 20 μg / ml of the reference substance;
[0053] c. Preparation of the test solution: accurately measure 1.0ml of the injection, put it in a 10ml measuring bottle, and distill it to volume;
[0054] d. Determination method: Precisely draw 10 μl each of the two concentrations of the reference substance solution and the test solution of the injection solution, respectively inject into the liquid chromat...
Embodiment 2
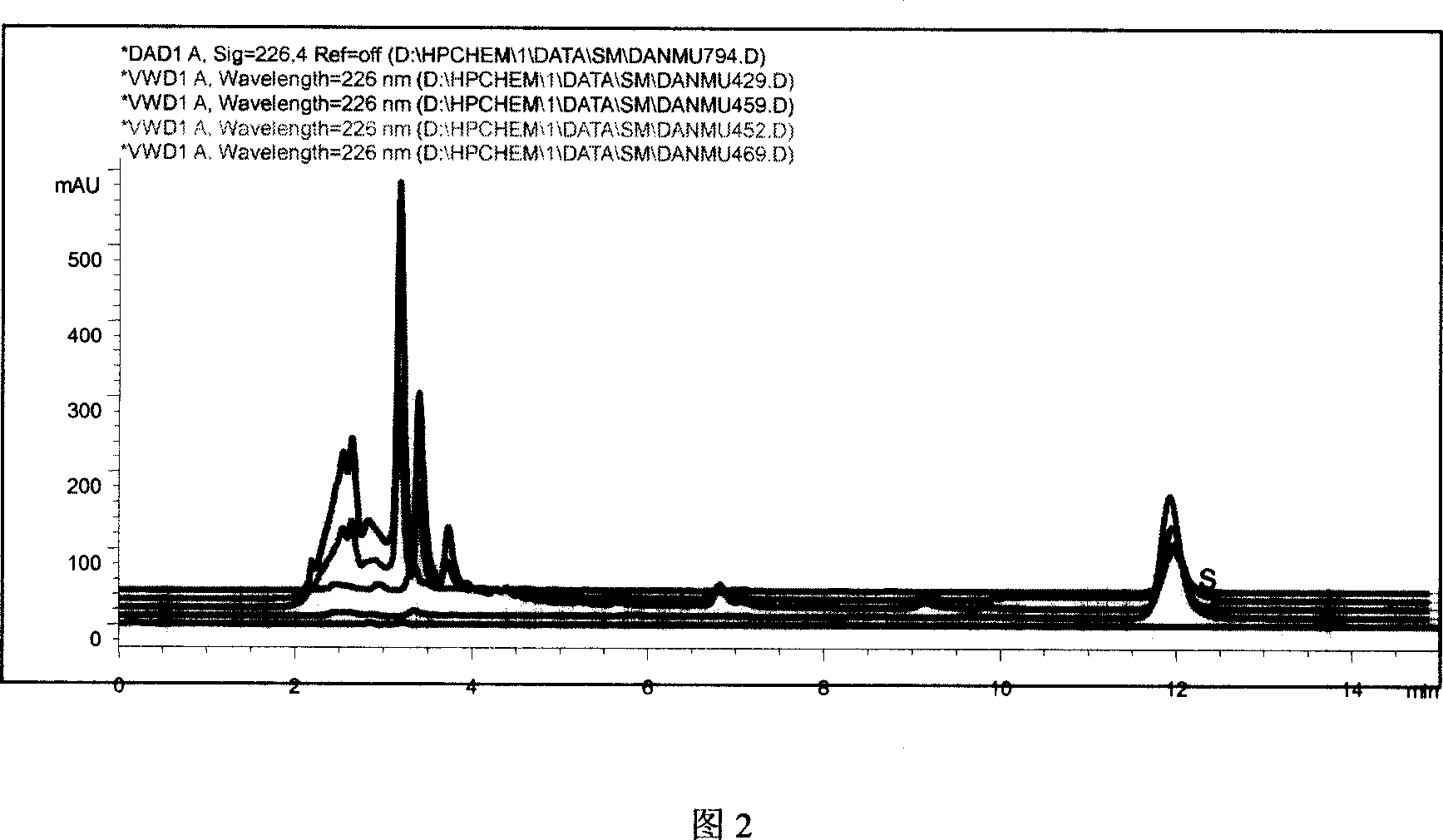
[0055] Example 2. A quality control method for Danmu injection, which is detected in combination with high performance liquid chromatography and fingerprints, the steps are as follows,
[0056] (1) Establish the standard fingerprint of Danmu injection,
[0057] a. Chromatographic conditions and system suitability test: Agilent 1100 series HPLC chromatograph, including binary high pressure pump, automatic sampling system, online degassing, column oven, DAD detector and other units; chromatographic column: Lichrospher C 18 , 5μm, 4.6×250mm; mobile phase: A is 0.1% phosphoric acid solution, B is 0.1% phosphoric acid acetonitrile solution, from 1% B to 35% B in 70min in a linear gradient, and then to 95% B in 5min, the flow rate : 1ml / min; Column temperature: 30°C; Detection wavelength: 226nm; Recording time: 72min;
[0058] Number of theoretical plates according to isovinblastam peak C 26 h 30 N 2 o 8 calculation, it should not be less than 5000;
[0059] b. Preparation of...
Embodiment 3
[0063] Example 3. The quality control method of the Danmu injection of embodiment 2, when establishing the standard fingerprint of the Danmu injection, at least 10 batches of the Danmu injection were taken, and the test solution was prepared according to the preparation method of the tested solution respectively. The above-mentioned acetonitrile is chromatographically pure, phosphoric acid is analytically pure, and water is ultrapure water. According to the detection data of at least 10 batches of Danmu injection obtained from the detection, the HPLC standard spectrum of Danmu injection is established, and the peak retention time and peak area are mainly established. Then import the detection data into Excel, calculate the average value of the relative retention time of each corresponding peak and the average value of the relative peak area, and multiply them with the average retention time and average peak area of the reference peak respectively, as the peak retention of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com