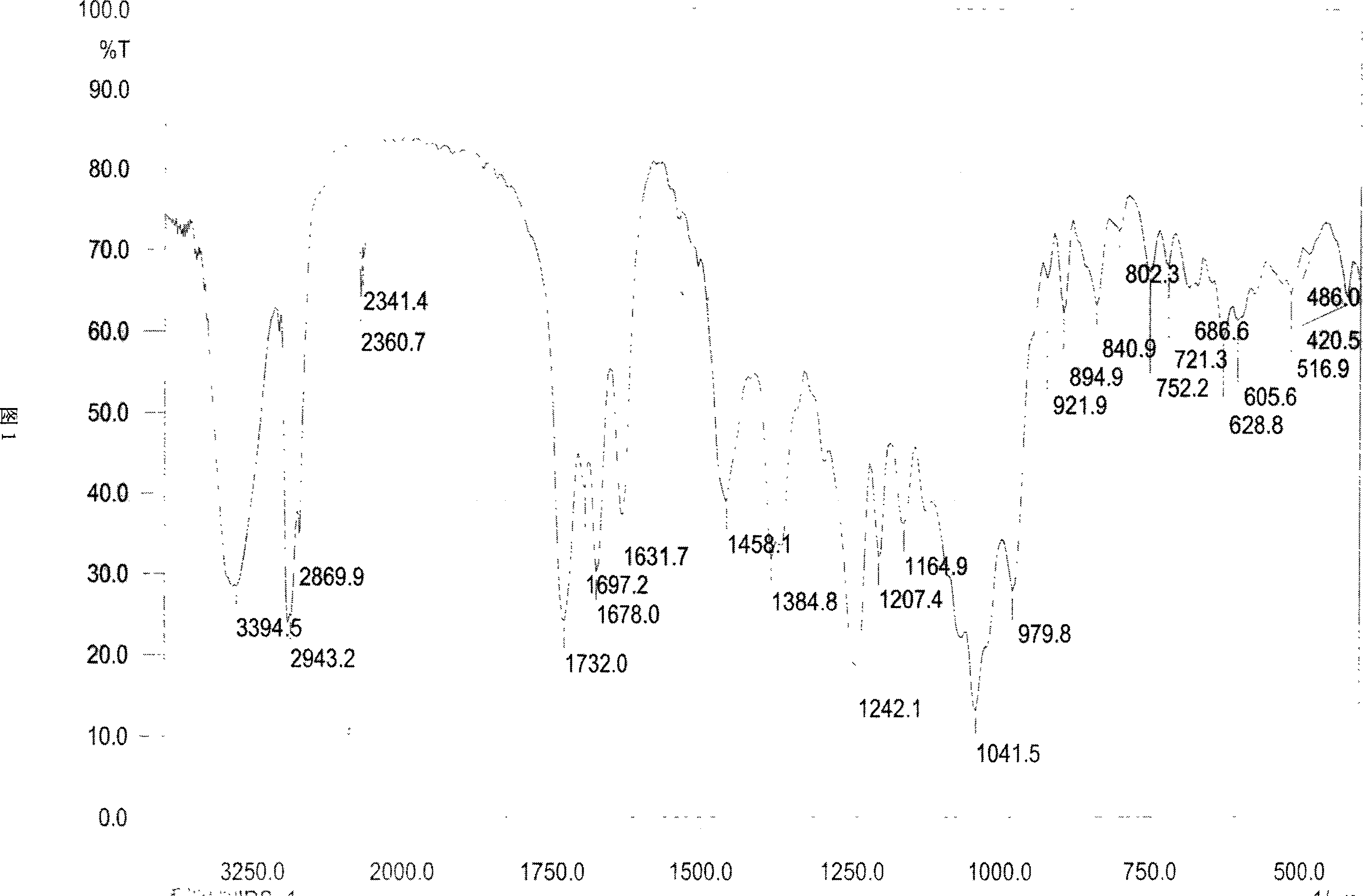
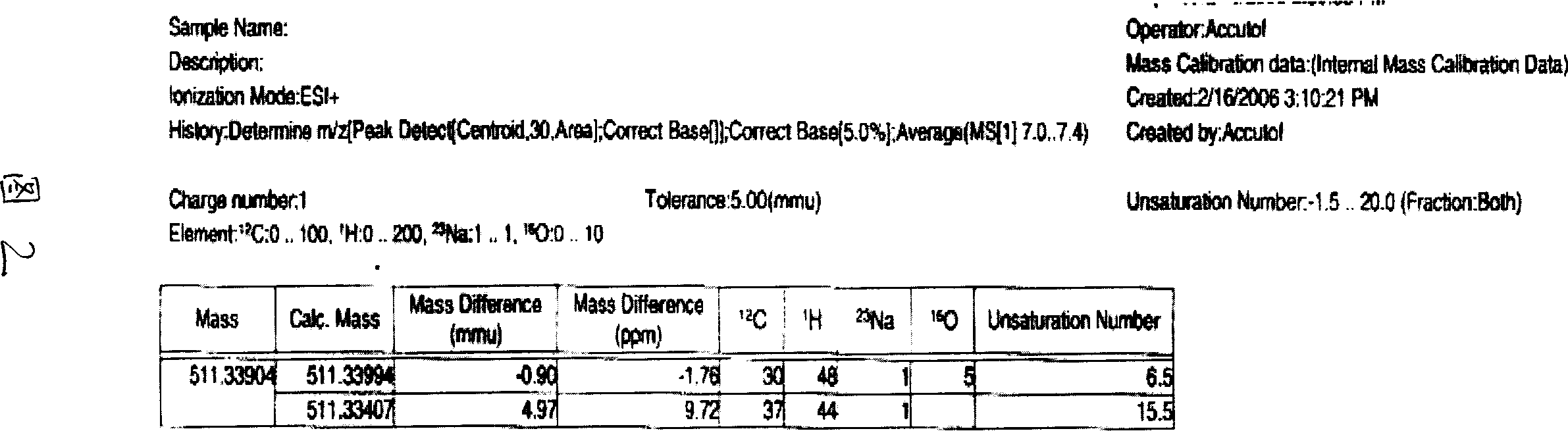
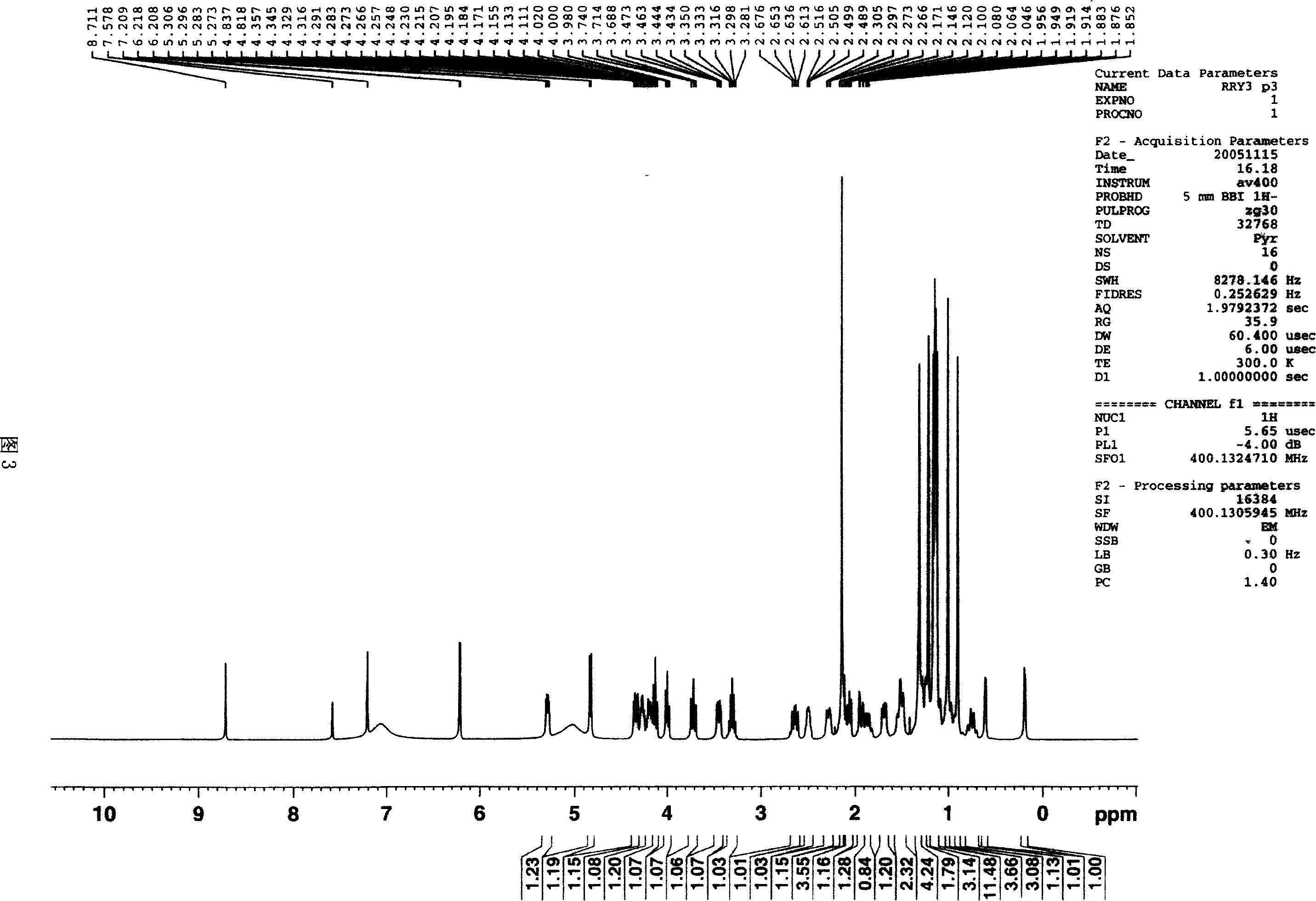
Triterpene saponin compound of cycro jackfruit alkyl, and effect for anti tumor
A technology of cyclopine and triterpene saponins, which is applied in the fields of antineoplastic drugs, steroids, organic chemistry, etc., to achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of compound 1
[0045] Take 4.0Kg of the rhizome of Actaea asiatica Hara, and extract it three times with 20L of 70% ethanol under reflux, each time for three hours; after combining the three extracts, recover the solvent to obtain the extract. Dissolve the extract in 1L of water, extract with 1L of petroleum ether, 4L of ethyl acetate, and 4L of n-butanol in sequence to obtain 300 g of ethyl acetate extract. The ethyl acetate extract was subjected to low-pressure column chromatography on silica gel (200-300 mesh), and eluted with petroleum ether-acetone-methanol system gradient (1:0:0-9:1:0-8:2:0.2-7: 2.5:0.5-6:3:1-4:4:2), 5000ml each, collected and combined into 5 fractions, of which the first fraction (102g) was subjected to silica gel (200-300 mesh) low-pressure column chromatography again, Gradient elution with chloroform-methanol system (10:0-9:1-8:2-7:3-6:4-5:5), 5000ml each, collected 9 fractions in total. Among them, the seventh ...
Embodiment 2
[0047] Tablet preparation
[0048] Formula I compound 20g
[0049] Lactose 80g
[0050] Magnesium Stearate 1.0%
[0051] 3% povidone ethanol solution appropriate amount
[0052] A total of 1000 capsules were made
[0053] Take the prescription amount of compound 1 and mix the prescription amount of lactose evenly, add 3% povidone ethanol solution to make soft material, pass through 18 mesh sieve to make granules, dry at 50°C for 30-45 minutes, granulate, add magnesium stearate, mix well , Tablets. Each tablet contains 20mg of the compound.
Embodiment 3
[0055] Preparation of capsules
[0056] Formula I compound 20g
[0057] Lactose 80g
[0058] Magnesium Stearate 1.0%
[0059] 3% povidone ethanol solution appropriate amount
[0060] A total of 1000 capsules were made
[0061] Take prescription amount of compound of formula I and the prescription amount of lactose, mix evenly, add 3% povidone ethanol solution to make soft material, pass through 18 mesh sieve to make granules, dry at 50°C for 30-45 minutes, granulate, add magnesium stearate, mix Evenly, fill in No. 1 capsule. Each capsule contains 20mg of the compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com