Use of phthalimide derivative in preparation of medicament for resisting angiogenesis
A new angiogenesis and use technology, applied in anti-tumor drugs, drug combinations, cardiovascular system diseases, etc., can solve the problems of unpublished, poor water solubility, and high medicinal toxicity, and achieve the effect of inhibiting the proliferation of vascular endothelial cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
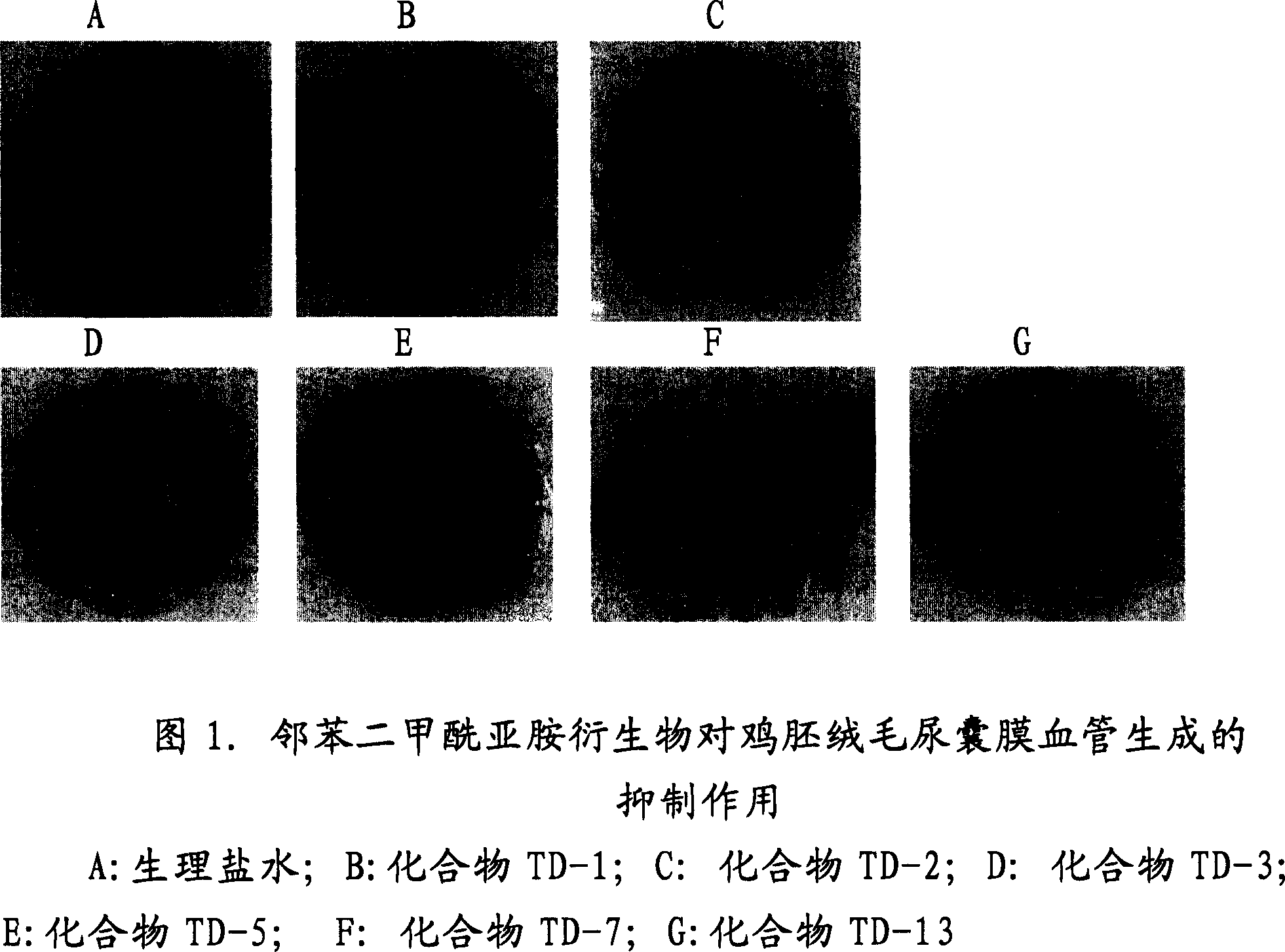
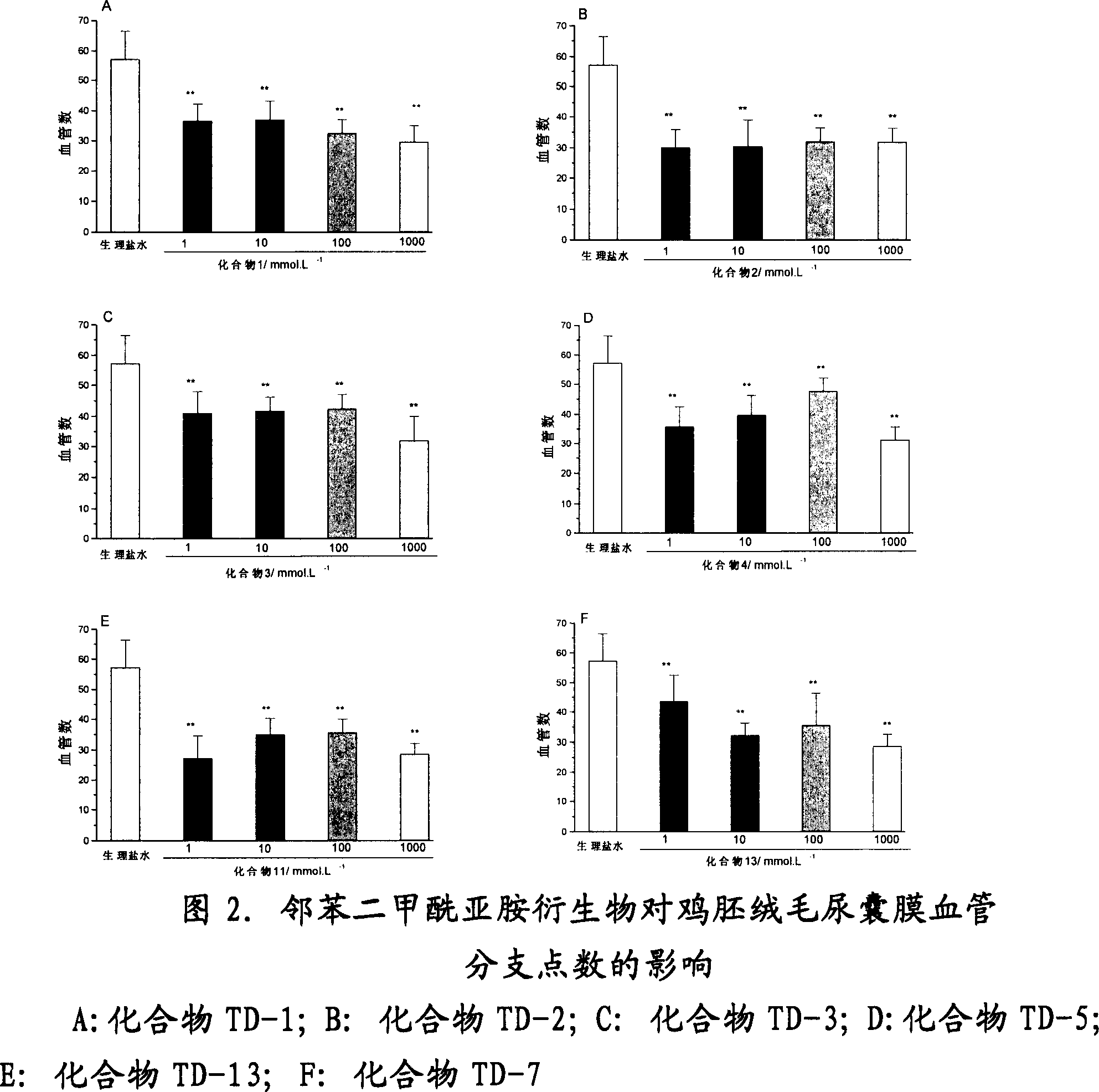
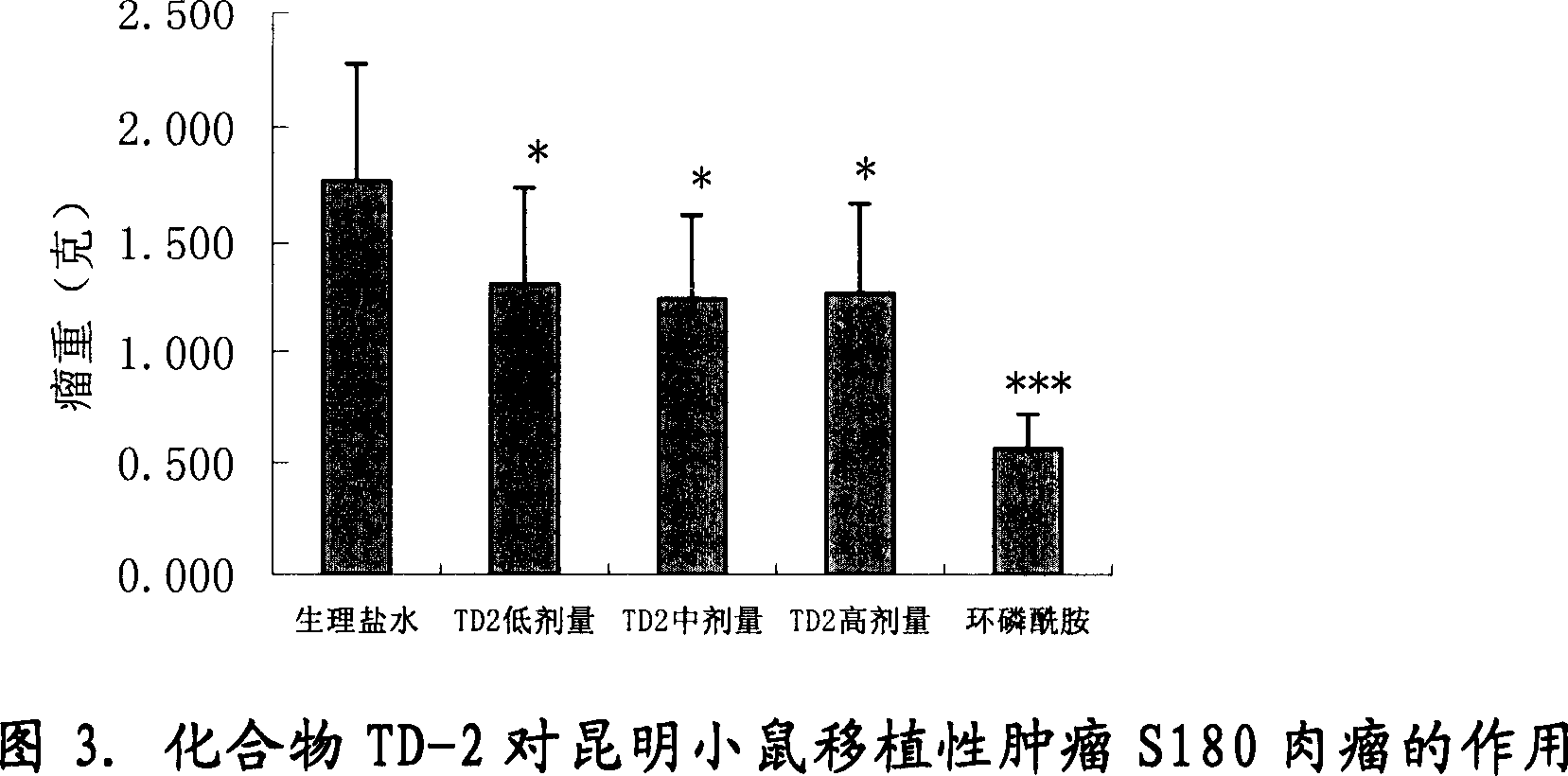
Image
Examples
Embodiment 1
[0046] Embodiment 1: Preparation of phthalimide derivatives (TD-1 to TD-18) of general formula I
[0047] Secondary amines (0.05 mol) were dissolved in 10 ml of dry benzene, and slowly dropped into dry benzene containing N-(bromoalkyl)-phthalimide (0.01 mol). After dripping, stir and reflux for 24h. 50ml of water was added to the reaction solution, and the layers were shaken, the benzene layer was separated, 10% hydrochloric acid was added, the water layer was separated and extracted with benzene (20ml×3). The aqueous layer was neutralized with 1N sodium hydroxide solution to pH 11, extracted with ether, combined with ether solution, washed with saturated sodium chloride solution and water in turn until neutral, dried overnight with anhydrous sodium sulfate, filtered, and hydrochloric acid was added to the filtrate After diethyl ether solution, a large amount of white precipitates were formed immediately. After filtration, the white precipitates were recrystallized (ethanol: ...
Embodiment 2
[0049] Example 2: (±)-N-(2-dimethylamino-ethyl)-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H )-Diketone hydrochloride (TD-19) preparation.
[0050] Take 2g of 1-chloro-2-dimethylamino-ethane hydrochloride, add 5ml of water to dissolve, ice bath, add 10% sodium hydroxide solution, adjust the pH value to 11, extract with ether (10ml×3), and combine the extracts , dried over night with anhydrous sodium sulfate, filtered, ether solution was removed under reduced pressure and low temperature (20-25°C), during this process, a small amount of solid appeared, which was the self-reaction product of 1-chloro-2-dimethylamino-ethane, Filter off the solid, add 20ml of dioxane, (±)-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione ( Lilidomide) 0.26g, anhydrous potassium carbonate 0.27g, stirred and refluxed for 2h, filtered, added 20ml of water in the filtrate, after shaking and layering, separated the dioxane layer, added 20ml of 10% hydrochloric acid, separated the aqueous layer...
Embodiment 3
[0051] Example 3: (±)-N-(2-diethylamino-ethyl)-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H ) - Preparation of diketone hydrochloride (TD-20).
[0052] Take 3g of 1-chloro-2-diethylamino-ethane hydrochloride and add 5ml of water to dissolve it. The solution is lavender red, put it in ice bath, add 10% sodium hydroxide solution, adjust the pH value to 11, extract with ether (10ml× 3), combine the extracts, dry overnight with anhydrous sodium sulfate, filter, the filtrate is light yellow, ether solution is removed under reduced pressure and low temperature (20-25°C), during this process, a small amount of white solid appears, which is 1-chloro- 2-Diethylamino-ethane self-reaction product, filter out the solid, add 20ml of dioxane, (±)-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole- 0.26g of 1,3(2H)-diketone (thalidomide) and 0.27g of anhydrous potassium carbonate were stirred and refluxed for 2h. The purification method is the same as TD-19. A large amount of white precipitate w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com