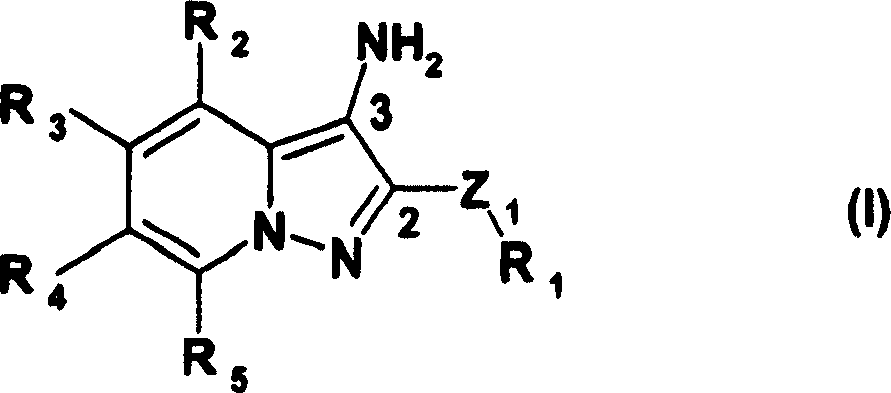
Composition comprising at least one 3-amino-pyrazolopyridine derivatives
A composition and pyrazolo technology, applied in the field of dyeing keratin fibers, especially human keratin fibers such as hair, can solve the problems of inability to obtain good resistance of chromaticity properties, limited range of hues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
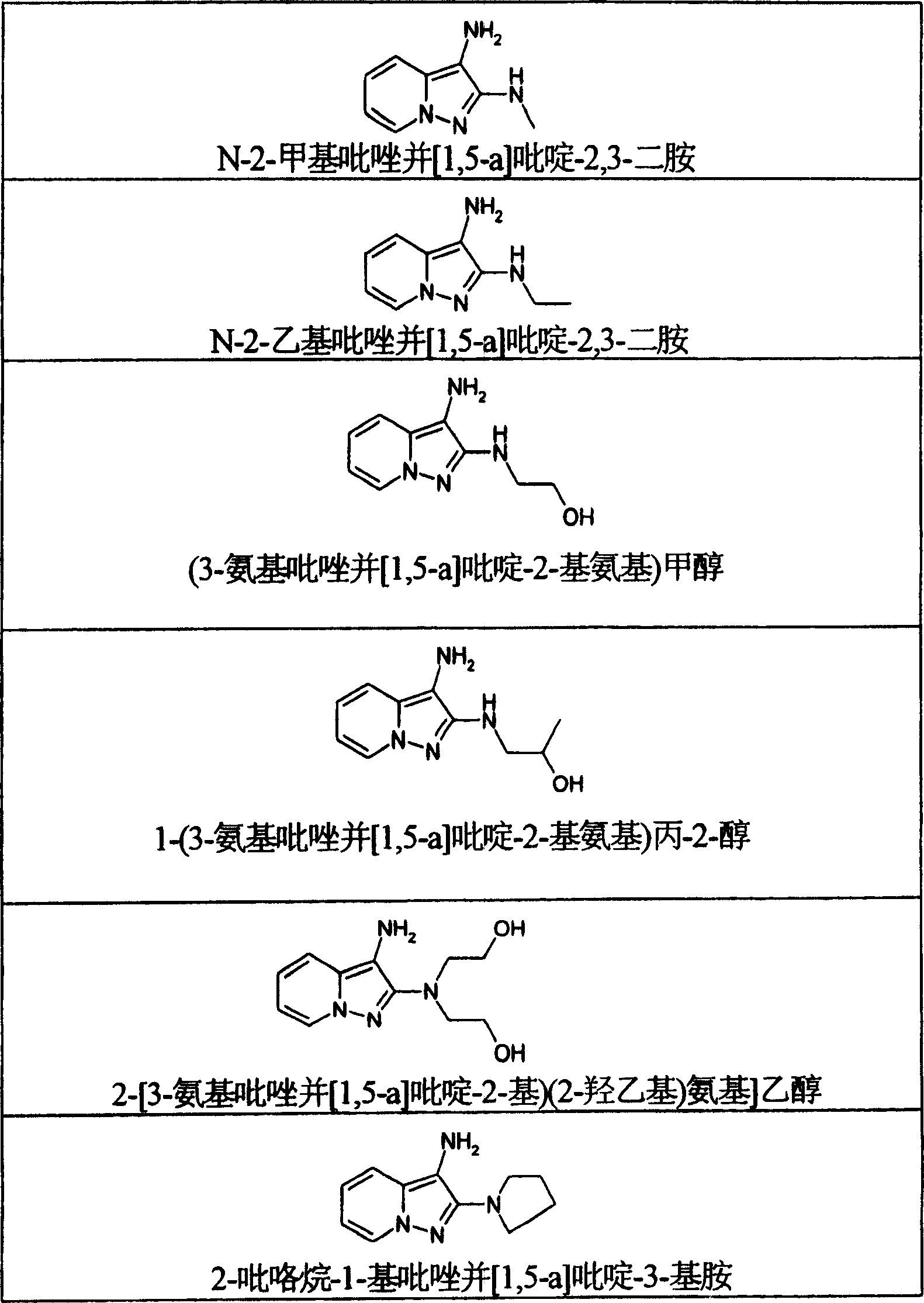
[0127] Example 1: N-2-Ethylpyrazolo[1,5-a]pyridine-2,3-diamine dihydrochloride
[0128]
[0129] Synthesis of 2-Methylthio-3-nitro-pyrazolo[1,5-a]pyridine
[0130]
[0131] In a 2-liter three-necked flask equipped with a mechanical stirrer and an internal temperature sensor, 111 g of 1-N-aminopyridine_iodide (0.5 mol) solution in DMF (500 rl) was prepared under nitrogen.
[0132] Then potassium carbonate (207.3, 3 equivalents) was added in one portion, followed by 1,1-bis(methylthio)-2-nitroethylene (165.2 g, 2 equivalents) in one portion. The weight of the reaction mixture increases. Then 500ml DMF was added to make the reaction mixture more fluid.
[0133] After stirring at room temperature for 48 hours, the reaction mixture was poured into 4 liters of ice water. The precipitate formed was filtered, washed with a large amount of water (5 liters), and then dried under vacuum at 80°C.
[0134] Excessive 1,1-bis(methylthio)-2-nitroethylene (30mol.%, by re-pasting) was removed...
Embodiment 2
[0156] Example 2: 2-(3-Amino-pyrazolo[1,5-a]pyridin-2-ylamino)ethyl acetone dihydrochloride
[0157]
[0158] Synthesis of 2-[(3-nitro-pyrazolo[1,5-a]pyridin-2-yl)amino]ethanol
[0159]
[0160] Add 10ml of N-methylpyrrolidone, 7.25g (0.03mol) of 2-methanesulfonyl-3-nitropyrazolo[1,5-a]pyridine and 6ml of ethanolamine into a spherical condenser, thermometer and magnetic stirring In a 50ml three-necked flask. Stir and heat to 70°C for 5 hours on an oil bath.
[0161] The yellow compound separated by pouring the reaction mixture into water was drained on the frit, and then washed several times with water. At P 2 O 5 After vacuum drying in the presence, 6.45 g of a yellow solid consistent with the expected compound was recovered.
[0162] NMR analysis ( 1 H 400MHz and 13 C100.61MHz DMSO d 6 ) Consistent with the expected structure.
[0163] The expected molecule C is mainly detected 9 H 10 N 4 O 3 Quasi-molecular ion [M+H] + , [M+Na] + And [M-H] - .
[0164] Synthesis of 2-(3-...
Embodiment 3
[0172] Example 3: 1-(3-Amino-pyrazolo[1,5-a]pyridin-1-ylamino)-propan-2-cetanol
[0173]
[0174] Synthesis of 1-[(3-nitropyrazolo[1,5-a]pyridin-2-yl)amino]propan-2-ol
[0175]
[0176] Add 10ml of N-methylpyrrolidone, 7.23g (0.03mol) of 2-methanesulfonyl-3-nitropyrazolo[1,5-a]pyridine and 7ml of 1-amino-2-propanol to the ball Condenser, thermometer and magnetic stirrer in a 50ml three-necked flask. While stirring, heat to 70°C for 5 hours on an oil bath.
[0177] Drain the yellow compound separated by pouring the reaction mixture into water on the frit, and then wash with water several times. At P 2 O 5 After vacuum drying in the presence, 6.02 g of a yellow solid consistent with the expected compound was recovered.
[0178] NMR analysis ( 1 H 400MHz and 13 C 100.61MHz DMSO d 6 ) Consistent with the expected structure.
[0179] Synthesis of 1-3-amino-pyrazolo[ 1,5-a]pyridin-1-ylamino)-propan-2-ol dihydrochloride
[0180]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com