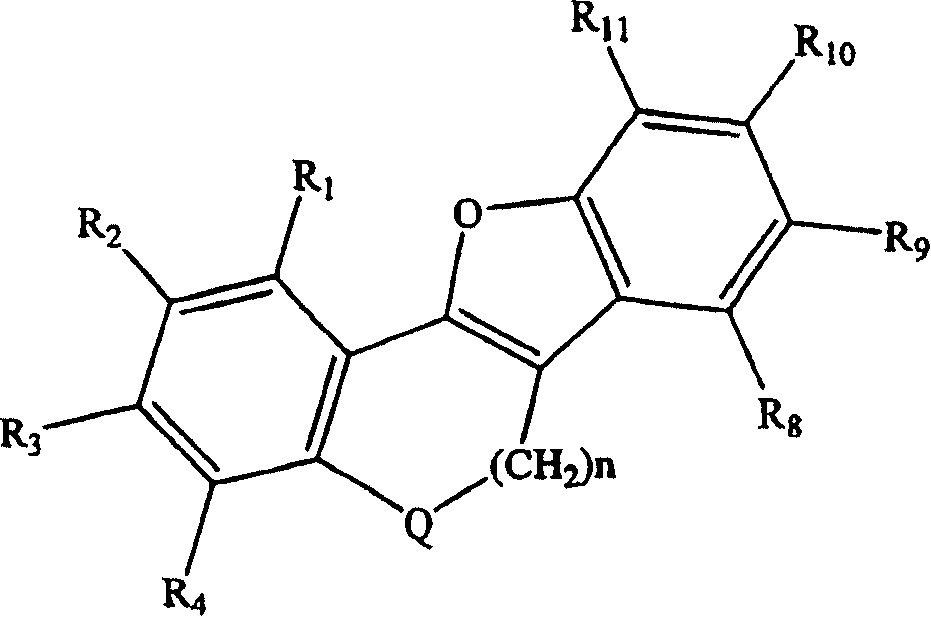
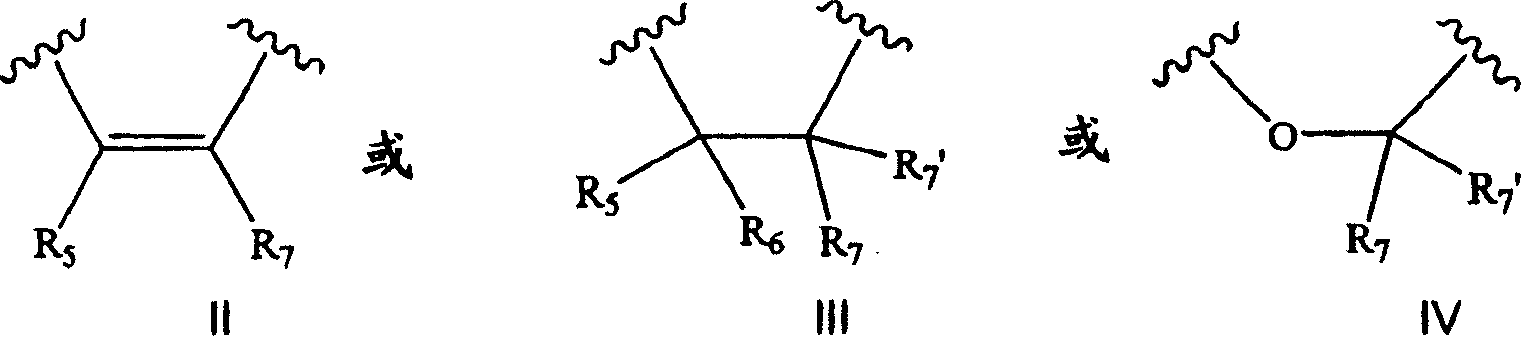
Tetracyclic compounds as estrogen ligands
A compound and halogen technology, applied in the direction of steroids, drug combinations, organic chemistry, etc., can solve problems such as unsatisfied estrogen substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0148] Synthesis of Example Compounds
[0149] The synthesis of the compounds described in the following examples is depicted in Reaction Schemes 1-9 below. The chemical preparation methods described herein can be monitored according to any suitable method known in the art. For example, product formation can be monitored by spectroscopic methods such as nuclear magnetic resonance spectroscopy (e.g. 1 H or 13 C), infrared spectroscopy, spectrophotometry (eg UV-visible), and mass spectrometry, or chromatography such as high performance liquid chromatography (HPLC) or thin layer chromatography.
[0150] Reaction scheme 1
[0151]
[0152] Reaction scheme 2
[0153]
[0154] Reaction Scheme 3 (preparation of stannane 13)
[0155]
[0156] Reaction scheme 4
[0157]
[0158] Reaction scheme 5
[0159]
[0160] Reaction scheme 6
[0161]
[0162] Reaction scheme 7
[0163]
[0164] Reaction Scheme 8 (Preparation of Precursor 30 Used in...
preparation Embodiment 1、2 and 3
[0169] Preparative Examples 1, 2 and 3 (from Reaction Scheme 1)
[0170] 2-Bromo-6-methoxy-3,4-dihydro-2H-naphthalen-1-one (3)
[0171] Dissolve 6-methoxy-1-tetralone 1 (100 g, 0.567 mol) in ether (2 liters), and add Br dropwise over 1 hour 2 (30ml, 0.59mol) for treatment. The solution was stirred for an additional 2 hours, then heated by adding 10% Na 2 SO 3 solution, NaHCO 3 Wash with brine for workup. The solution was allowed to stand overnight and 30 g of crystals were filtered over the next day. Concentration of the remaining solution afforded an additional 98 g of product. Combined yield of desired product was 128 g (88%). The resulting product was used as it is in the following reaction.
[0172] 2-Bromo-6-methoxy-3,4-dihydro-naphthalen-1-yl acetate (5)
[0173] A solution of 3 (80 g, 0.325 mol) in THF (200 mL) was cooled to -78 °C and treated by slowly adding 0.65 L of a 0.53 molar solution of LiHMDS in THF. The reaction...
Embodiment 2
[0180] Mp=219-220°C; MS
[0181] m / z 253(M+H) + .
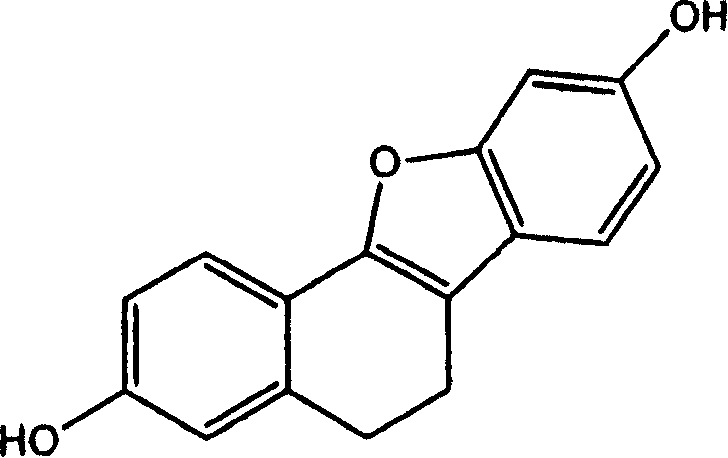
[0182] Benzo[B]naphtho[2,1-D]furan-3,9-diol (Example 2)
[0183] Example 1 (0.22 g, 0.00087 mol (based on 88% pure material)) was treated with DDQ (0.24 g, 0.001 mol) and heated to reflux in dioxane (20 mL) for 30 minutes. The reaction mixture was concentrated onto silica gel and purified by chromatography (EtOAc / hexanes; 3:7) to afford Example 2 (0.1 g, 46%):
[0184] Mp = 250-260°C; 1 H NMR (DMSO-d 6 )δ9.85(s, 1H), 9.80(s, 1H), 8.15(d, 1H, J=8.9Hz), 7.96(d, 1H, J=8.6Hz), 7.87(d, 1H, J=8.3 Hz), 7.63(d, 1H, J=8.6Hz), 7.30(d, 1H, J=1.9Hz), 7.23(dd, 1H, J=8.8Hz, 2.1Hz), 7.12(d, 1H, J= 1.9Hz), 6.88(dd, 1H, J=8.3Hz, J=1.9Hz).
[0185] 5-Bromo-benzo[B]naphtho[2,1-D]furan-3,9-diol (Example 3)
[0186] A solution of Example 2 (0.25 g, 10 mmol) and pyridine (0.79 g, 10 mmol) in dichloromethane (10 mL) was treated with acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com