Magnetically guided atherectomy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
second embodiment
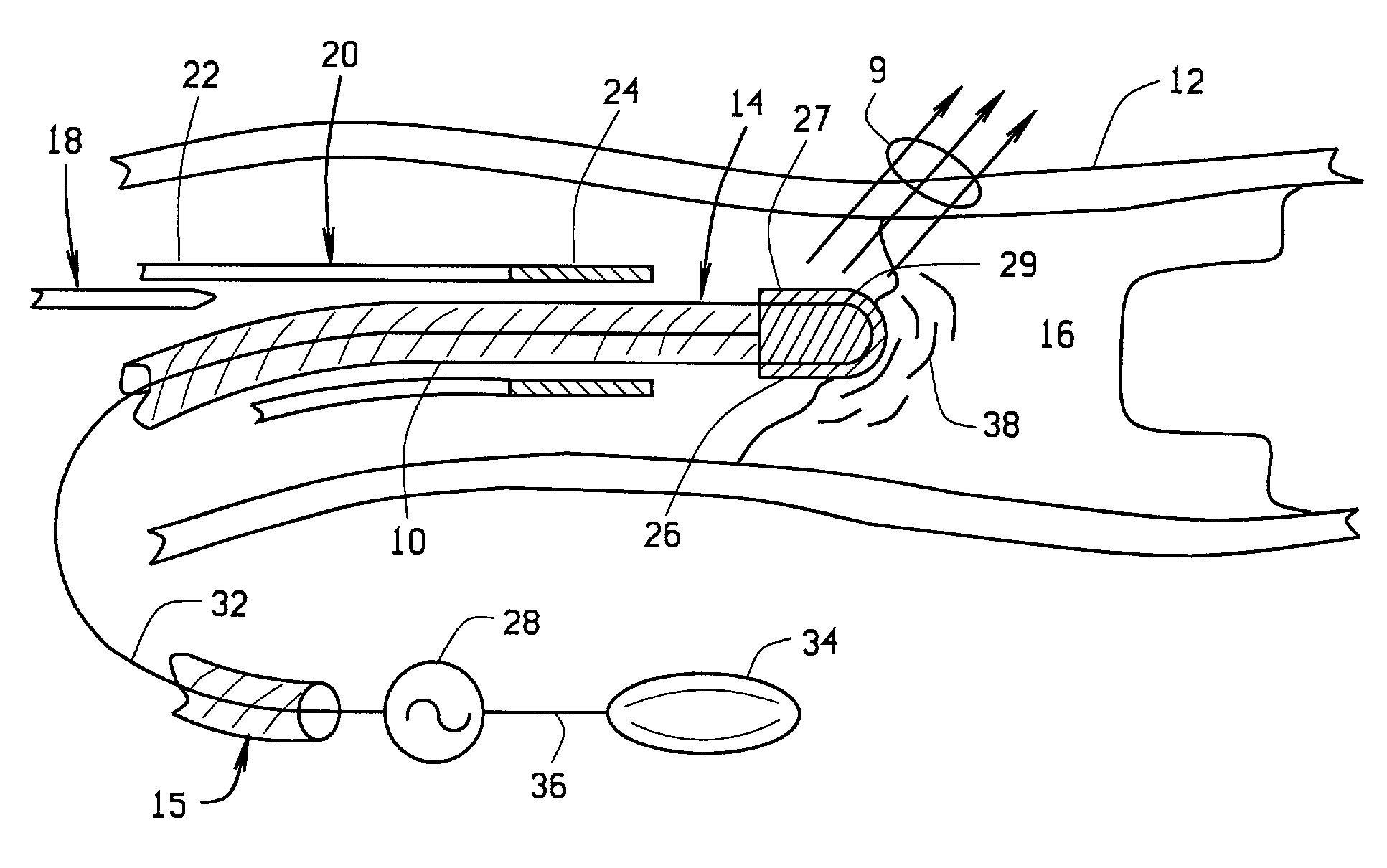
[0059] a magnetically guided atherectomy device is indicated generally as 400 in FIGS. 14-16. The magnetically guided atherectomy device 400 comprises an elongate catheter 402, having a proximal end (not shown) and a distal end 406, with a lumen 408 therebetween. The catheter 402 can be made of any flexible, biocompatible material conventionally used for medical catheters, for example Pebax.
[0060] A support 410 is mounted in the lumen adjacent the distal end 406. This support 410 can be permanently affixed within the catheter 402, with only the distal portion of the support projecting beyond the distal end 406 of the catheter. The support 410 is preferably made of a transparent, biocompatible material, such as polyethylene, polycarbonate, Pebax, or other suitable material. The support 410 includes passages for the ablation electrode conductor, and imaging, and preferably also includes compartments for receiving magnet body as described in more detail below.
[0061] The magnetically gu...
third embodiment
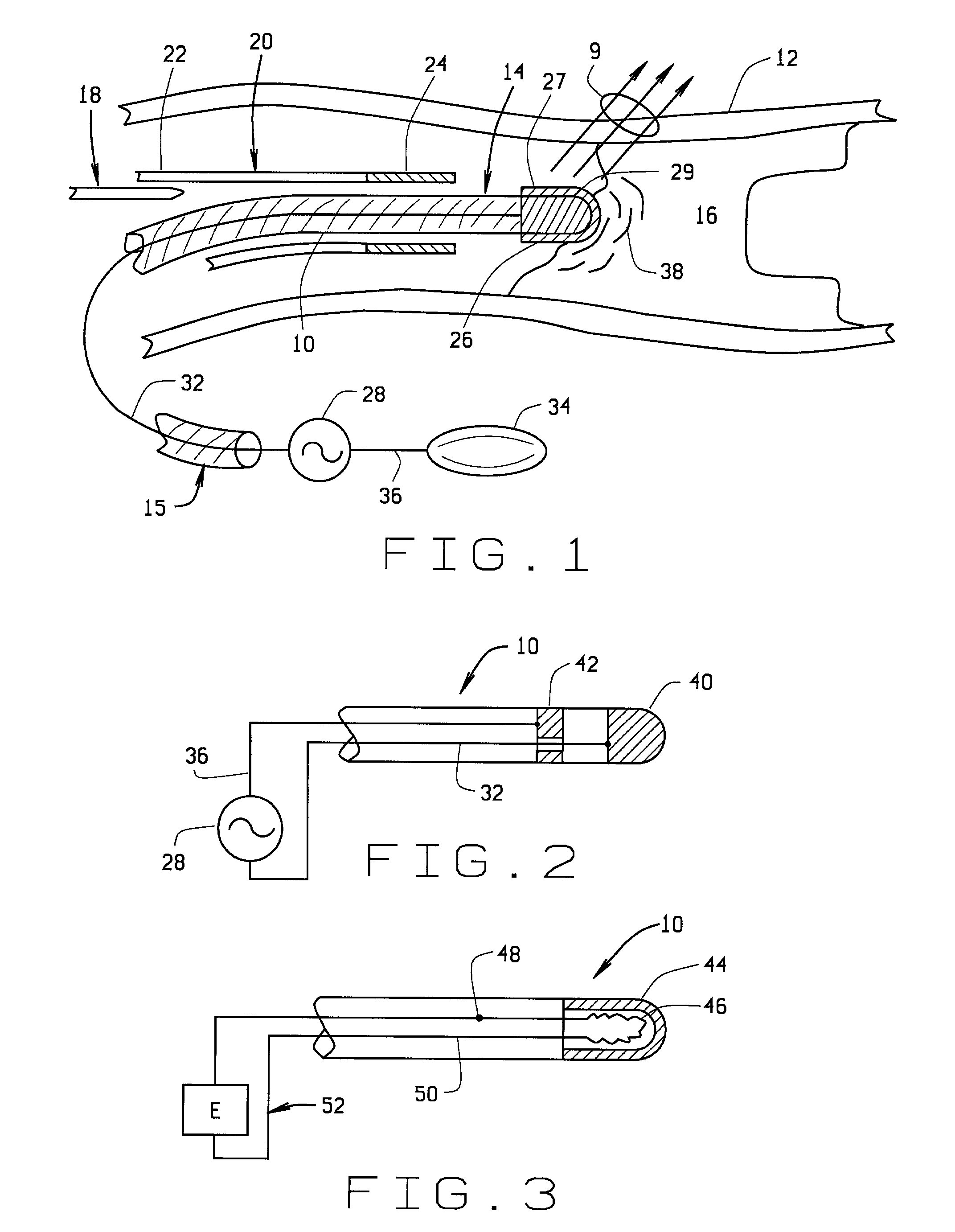
[0066] a magnetically guided atherectomy device is indicated generally as 500 in FIGS. 17-19. The magnetically guided atherectomy device 500 comprises an elongate catheter 502, having a proximal end (not shown) and a distal end 506, with a plurality of lumens 508 therebetween. The catheter 502 can be made of any flexible, biocompatible material conventionally used for medical catheters, for example Pebax, and is preferably transparent.
[0067] There is a disc-shaped electrode 510 on the distal end of the catheter 502. The electrode 510 has an opening 512 therein, generally transverse to the plane of the disc. A conduit 514 can extend through a generally central passageway 516 in the catheter 502 and through the opening 512 in the electrode 510, to make an electrical connection with the electrode, and provide energy to the electrode 510 for ablating atheramatous material that the electrode contacts. In this preferred embodiment the conduit 514 can be extended relative to the distal end...
fourth embodiment
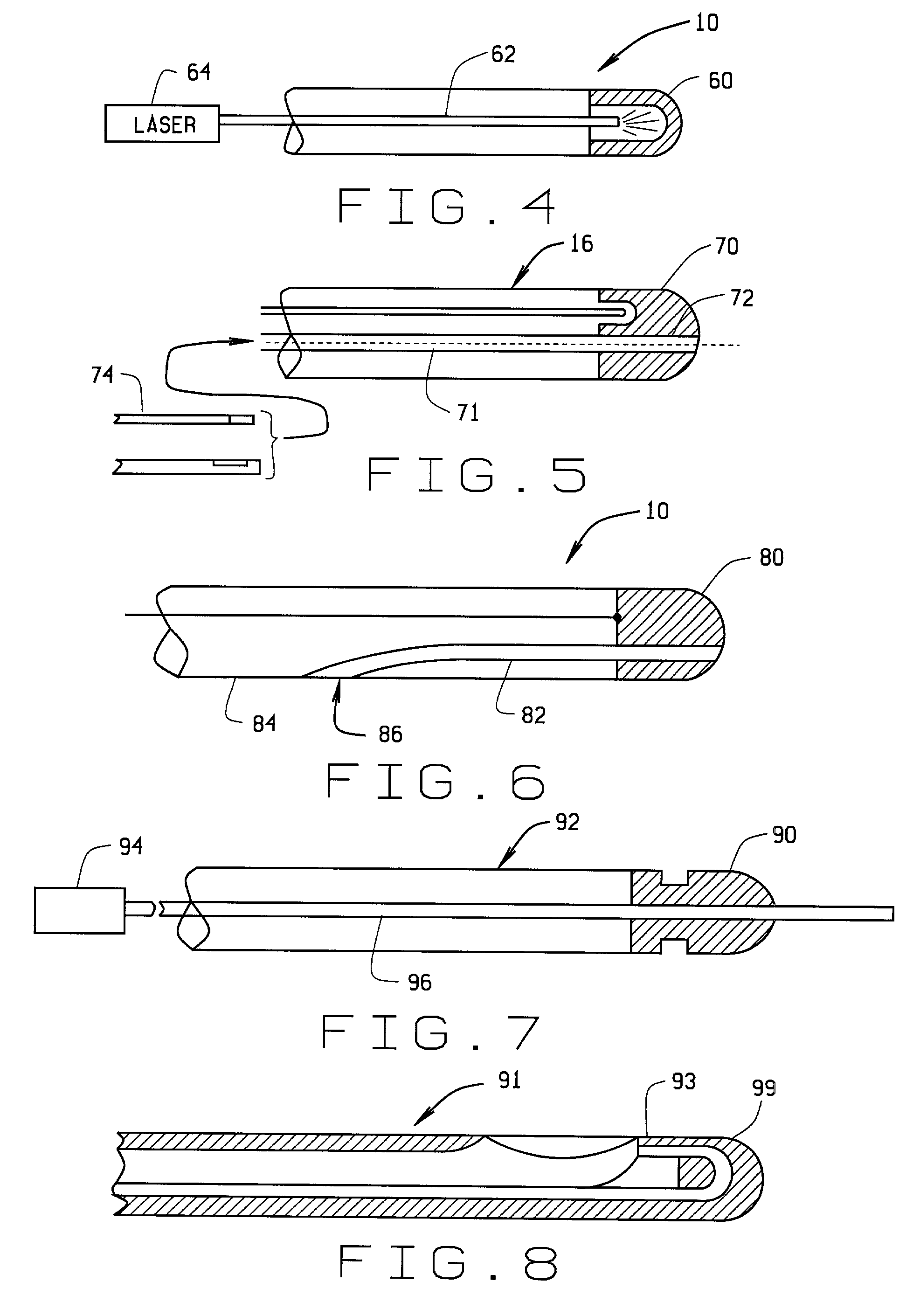
[0074] a magnetically guided atherectomy device is indicated generally as 600 in FIGS. 20-21. The magnetically guided atherectomy device 600 comprises an elongate catheter 602, having a proximal end (not shown) and a distal end 606, with at least one lumen 608 therebetween. The catheter 602 can be made of any flexible, biocompatible material conventionally used for medical catheters, for example Pebax, and is preferably transparent.
[0075] There is a dome-shaped cutting head 610 on the distal end of the elongate catheter 602. The cutting head 610 has a centrally opening an annular cutting edge 612 aligned with the lumen of the catheter. The smooth, dome shape allows distal end of the device to be manipulated within the blood vessel without damaging the inside structure of the blood vessels. The opening allows material that has been cored from the blood vessel to pass through the cutting head 610 to the lumen of the catheter where it can be accumulated or flushed out of the system.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com