Novel compounds having antitumor activity: process for their preparation and pharmaceutical compositions containing them
- Summary
- Abstract
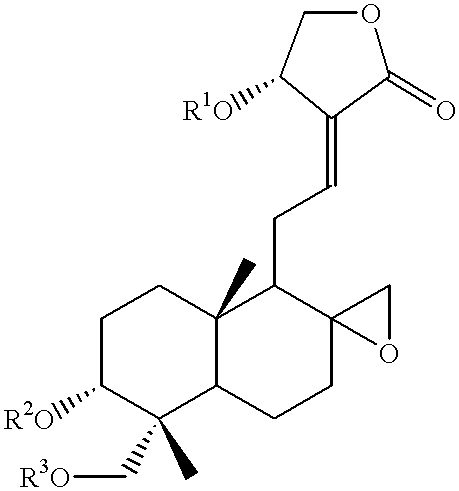
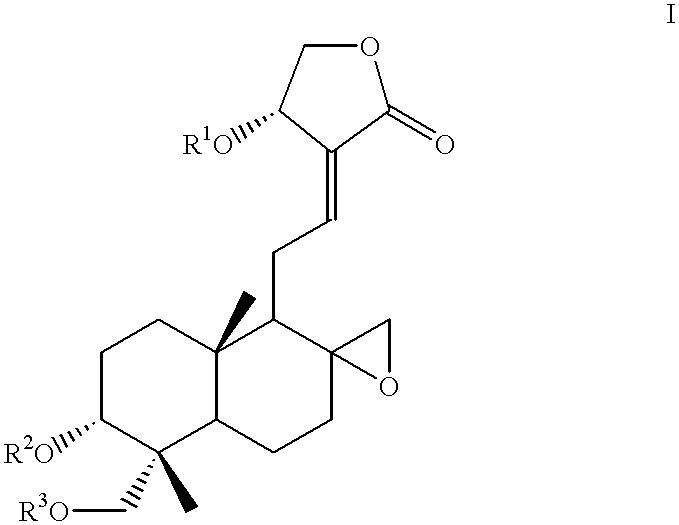
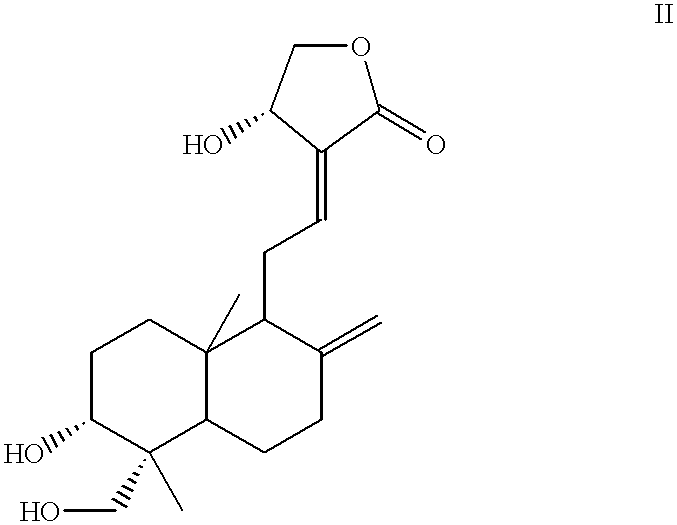
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of 3,14,19-Triacetyl 8,17-Epoxy Andrographolide
[0087] 14
[0088] 8,17-epoxy andrographolide (100 mg) obtained in Example 1 above was taken in 2 ml of acetic anhydride and refluxed for 5 minutes. The reaction was monitored by TLC. After completion of the reaction, the reaction mixture was diluted with solvent ether, washed with water and dried over Na.sub.2SO.sub.4 and concentrated. The residue obtained was chromatographed over a column of silicagel (60-120 mesh) with light petrol:ethyl acetate (65:35) as the solvent system to afford the title compound as a colourless solid (90 mg, 67%). mp: 195.degree. C.
[0089] .sup.1H NMR: .delta. 7.09 (1H, t, J=10 Hz, C-12H), 5.88 (1H, d, J=5.8 Hz, C-14H), 4.55 (1H, C-3H), 4.5(1H, C-15Ha), 4.29(1H,C-19Ha), 4.21 (1H, C-15Hb), 4.16(1H, C-19Hb), 2.6(2H, dd, J=12,4 Hz, C-17H), 2.11 (3H, S, OAc), 2.05(6H, s, OAc)
example 3
Preparation of 3,14,19-Tripropionyl 8,17-Epoxyandrographolide
[0090] 15
[0091] 8,17-epoxyandrographolide (200 mg) obtained in Example 1 was taken in propionic anhydride (3 ml) and the mixture was refluxed for 5 minutes. The reaction was monitored by TLC. After completion of the reaction, the reaction mixture was poured into water (50 ml) and extracted with dichloromethane. The organic layer was dried over Na.sub.2SO.sub.4 and concentrated. The concentrated residue was recrystallised with ethanol to yield the title compound as colourless solid (115 mg, 39%) m.p. 119.degree. C.
[0092] .sup.1H NMR: .delta. 7.1 (1H, t, J=10 Hz, C-12H), 5.9(1H, d, J=5.8 Hz, C-14H), 4.7-4.5 (m), 4.4-4.0 (m), 2.6 (2H, dd, J=12, 4 Hz, C-17H), 2.5-2.3 (m, Propyl)
example 4
Preparation of 3,14,19-Tris Chloro Acetyl 8,17-Epoxy Andrographolide
[0093] 16
[0094] 8,17-epoxy andrographolide (400 mg) obtained in Example 1, chloro acetic acid (770 mg), dicyclohexylcarbodiimide (1.6 gms) and triethyl amine (1 ml) were taken in dichloromethane (20 ml) and the mixture stirred for 1 hour at room temperature. The reaction was monitored by TLC. After completion of the reaction, the reaction mixture was filtered to remove the insoluble urea, diluted with dichloromethane, washed with saturated NaHCO.sub.3 and water successively. The organic layer was dried over Na.sub.2SO.sub.4 and concentrated. The residue obtained was chromatographed over a column of silicagel (60-120 mesh) with chloroform:acetone (98:2) as the eluent to yield the title compound as a colourless solid (200 mg, 31%) m.p 180.degree. C.
[0095] .sup.1HNMR: .delta. 7.1 (1H, t, J=10 Hz, C-12H), 6.0 (1H, d, J 5.8 Hz, C-14H), 4.7-4.5 (m), 4.4-4.0 (m), 2.6 (2H, dd,J=12, 4 Hz, C-17H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com