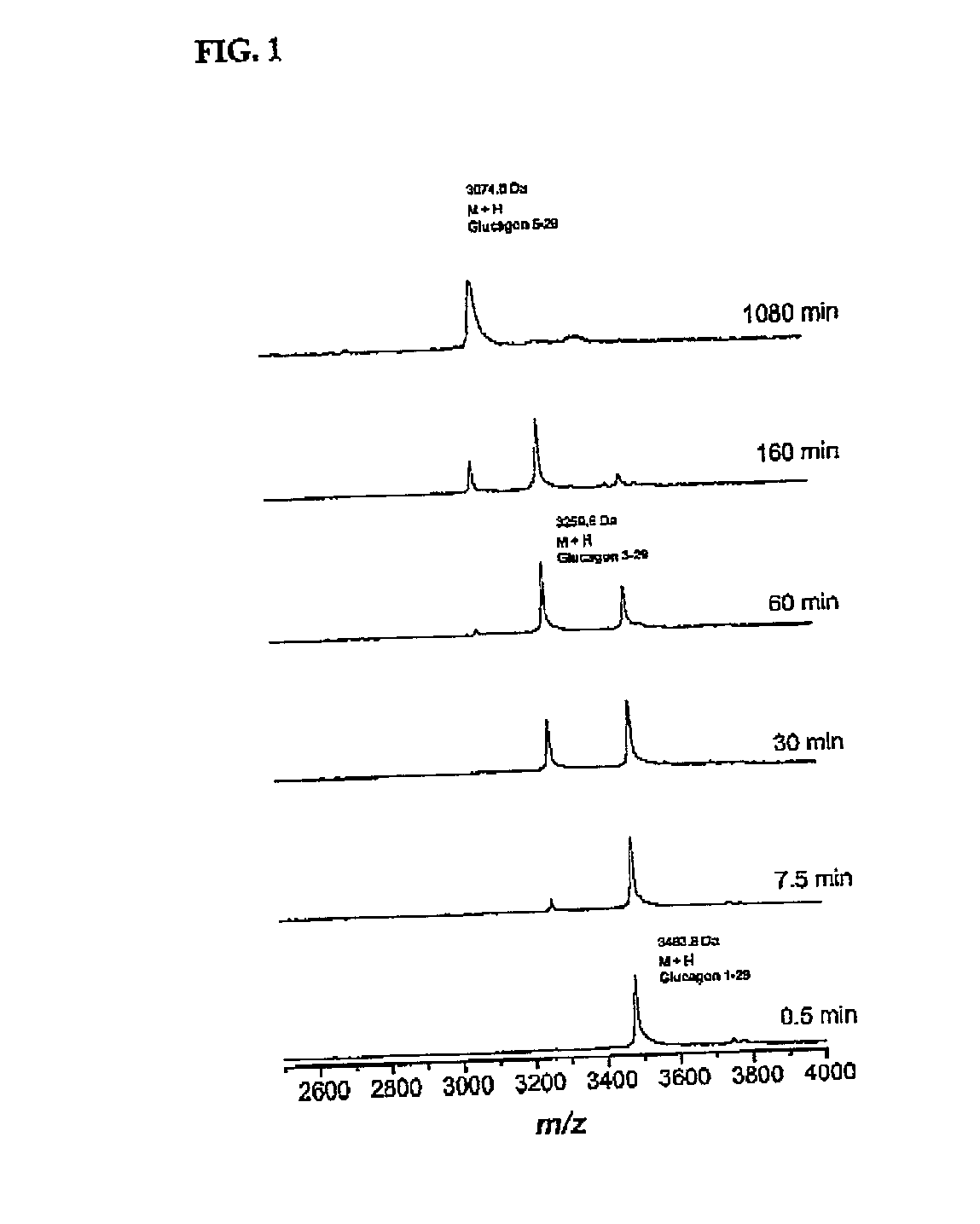
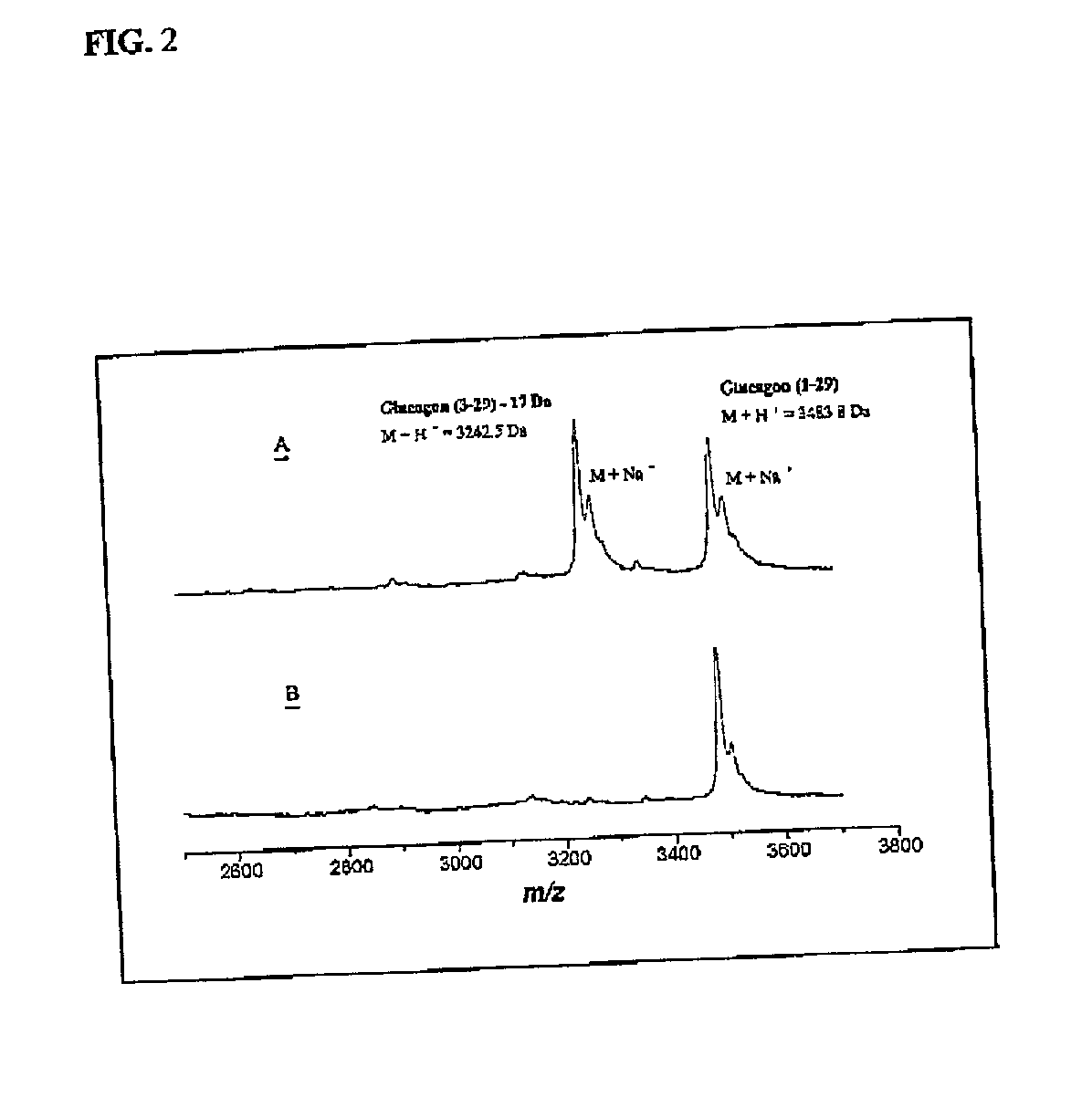
Method for raising the blood glucose level in mammals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
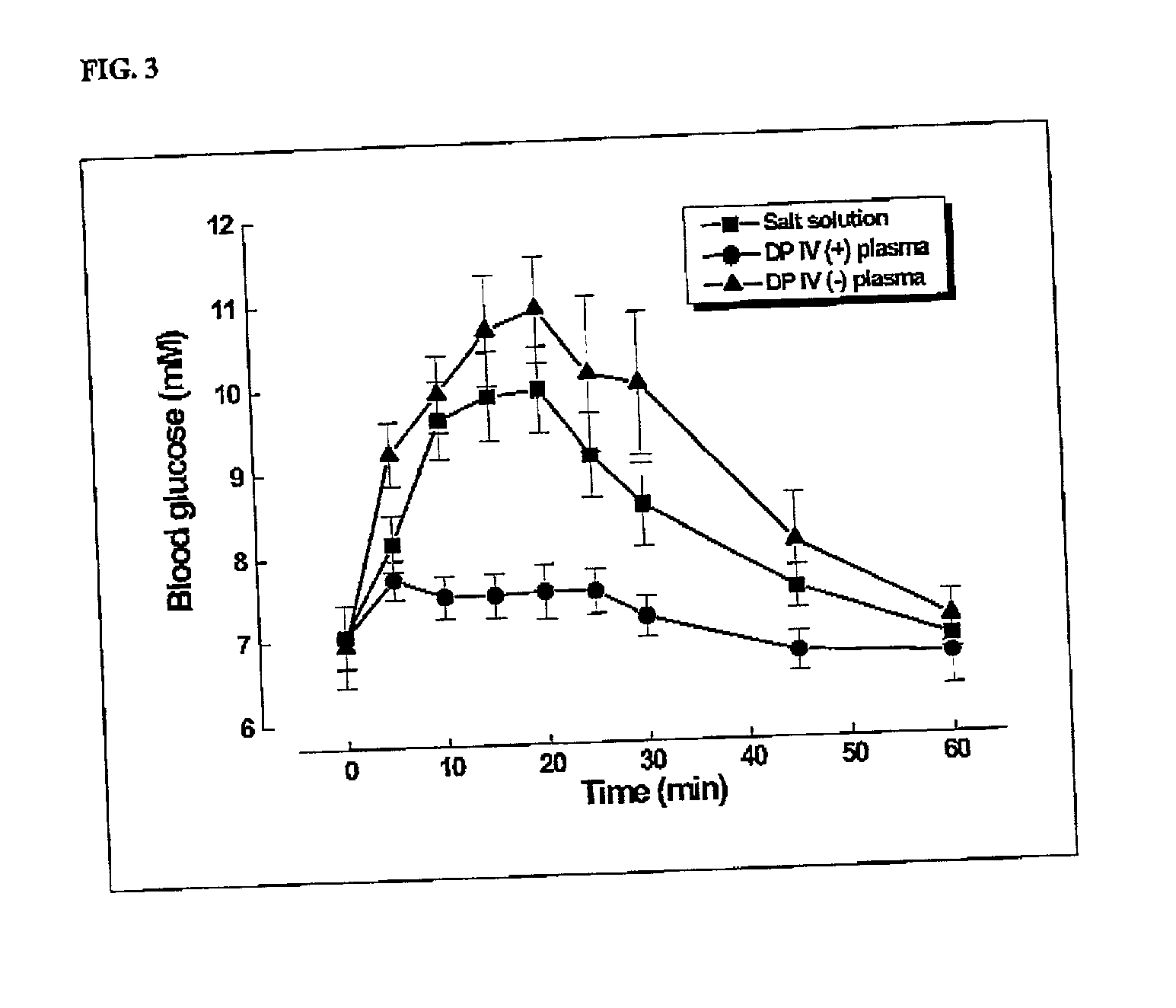
Effect of Glucagon on Endogenous Glucose Release After Incubation in Plasma of DP IV-Positive and DP IV Negative Rats
[0058] To test whether glucagon-degrading activity is present in the plasma of DP IV-negative rats, 6.8 .mu.g of glucagon were reinsulated for three hours at 37.degree. C. in 1.0 ml of plasma of normal, DP IV-positive rats and in 1.0 ml of plasma of DPrats. From 10 to 50 .mu.l of the incubation solution were injected i.v. into normal Wistar rats and compared with a saline control. The biological response, that is to say the increase in blood glucose resulting from the glucagon-stimurelease of hepatic glucose, was monitored for 60(FIG. 3).
example 3
Effect of Glucagon on Glucose Response in Wistar Rats After i.v. Injection of Preincubated Glucagon in the Plasma of a Normal Rat, in the Presence and Absence of DP IV Inhibitor
[0059] To test whether the effect of the glucagon-degrading activin plasma can be inhibited by a specific DP IV inhibitor, 6.8 .mu.g of glucagon were induced for three hours at 37.degree. C. in 1.0 of normal rat plasma and in 1.0 ml of normal rat plasma additionally containing 0.01 mmol of isoleucyl-thiazolidide. From 10 to 50 .mu.l of the incubation solution were injected iv. into normal Wistar rats and compared with a saline control. The biological response, that is to say the increase in blood glucose resulting from the glucagon-stimulated release of hepatic glucose, was monitored for 30(FIG. 4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com