Secretory immunoglobulin produced by single cells and methods for making and using same
a technology of secretory immunoglobulin and cultured cells, which is applied in the direction of antibody medical ingredients, immunological disorders, peptide/protein ingredients, etc., can solve the problems of inefficient formation of disulfide bonds between diga and sc in vitro, unstable non-secretory forms with relatively short half-lives, and limited therapeutic use, so as to avoid sig alterations, reduce hospitability, and be more efficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Cloning Human Ectoplasmic Domain
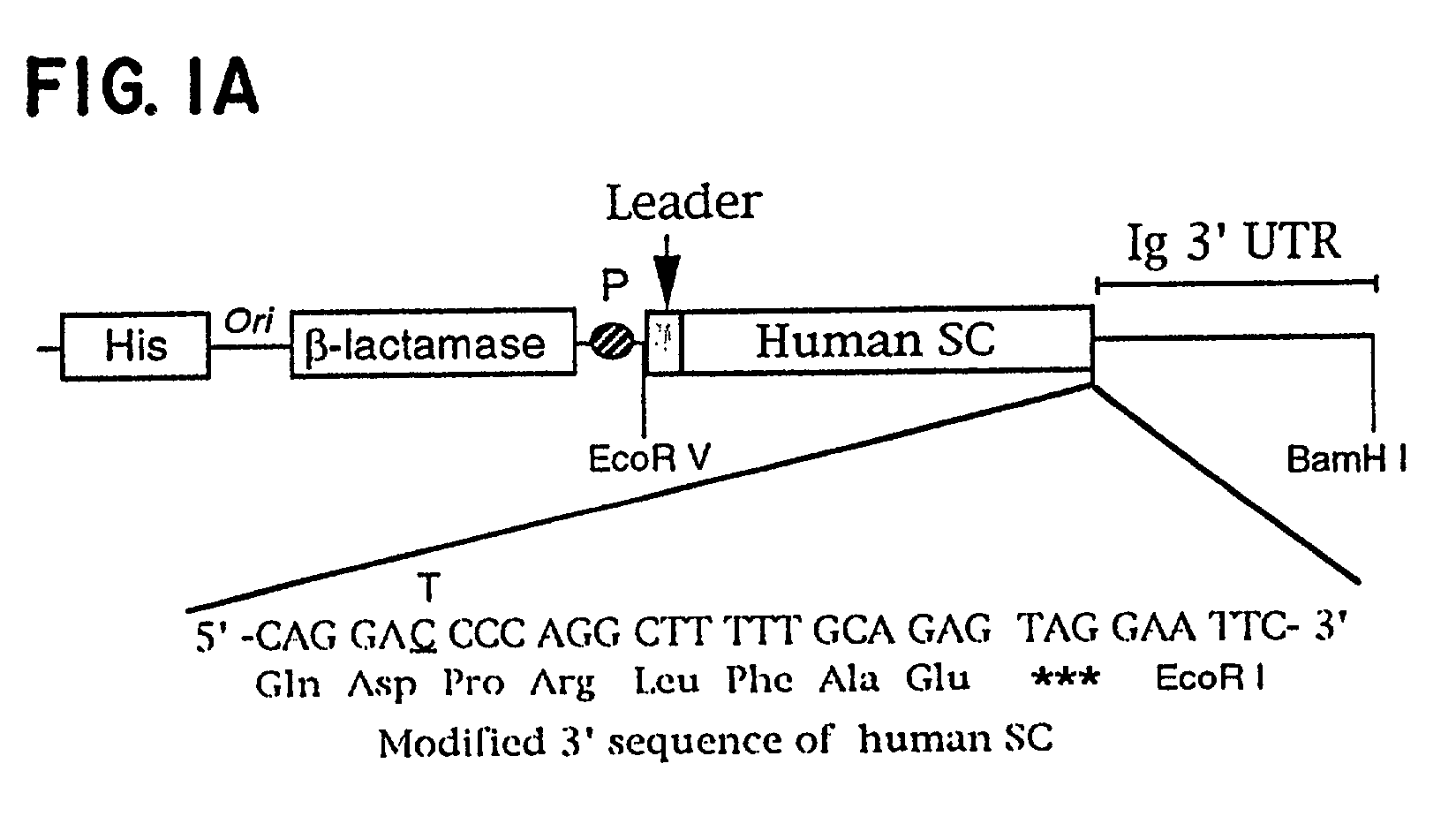
[0061] To produce sIgA a gene coding for human pIgR was obtained from Dr. Charlotte S. Kaetzel (University of Kentucky, Lexington, Ky.). A fragment from pIgR containing only the ectoplasmic domain (SC) and lacking the transmembrane and the cytoplasmic domains was generated. A 1402 bp PCR fragment was generated using the complete human pIgR cDNA in pBluescript (Tamer et al., Mol. Immunol. 30 413-421, 1993, this article and all other articles cited herein are incorporated herein by reference) as template and the primers:
1 1. 5'-GGGCAGAACGGTGACCATCAACTGCCCTTT-3' (SEQ ID NO:1) and 2. 5'-AAGGAATTCCTACTCTGCAAAAAGCCTGGGGTCCTGAATGGC-3' (SEQ ID NO:2)
[0062] The second primer included a silent base change upstream of Glu589 to delete a BamHI site in the SC coding region to facilitate cloning. A stop codon, shown by underlining, followed by an EcoRI site downstream of Glu589 were also included. A stop codon was introduced at amino acid 590, the position of normal...
example 2
Expression of Cloned Human Ectoplasmic Domain in Cells Secreting Mouse-Human Chimeric IgA1
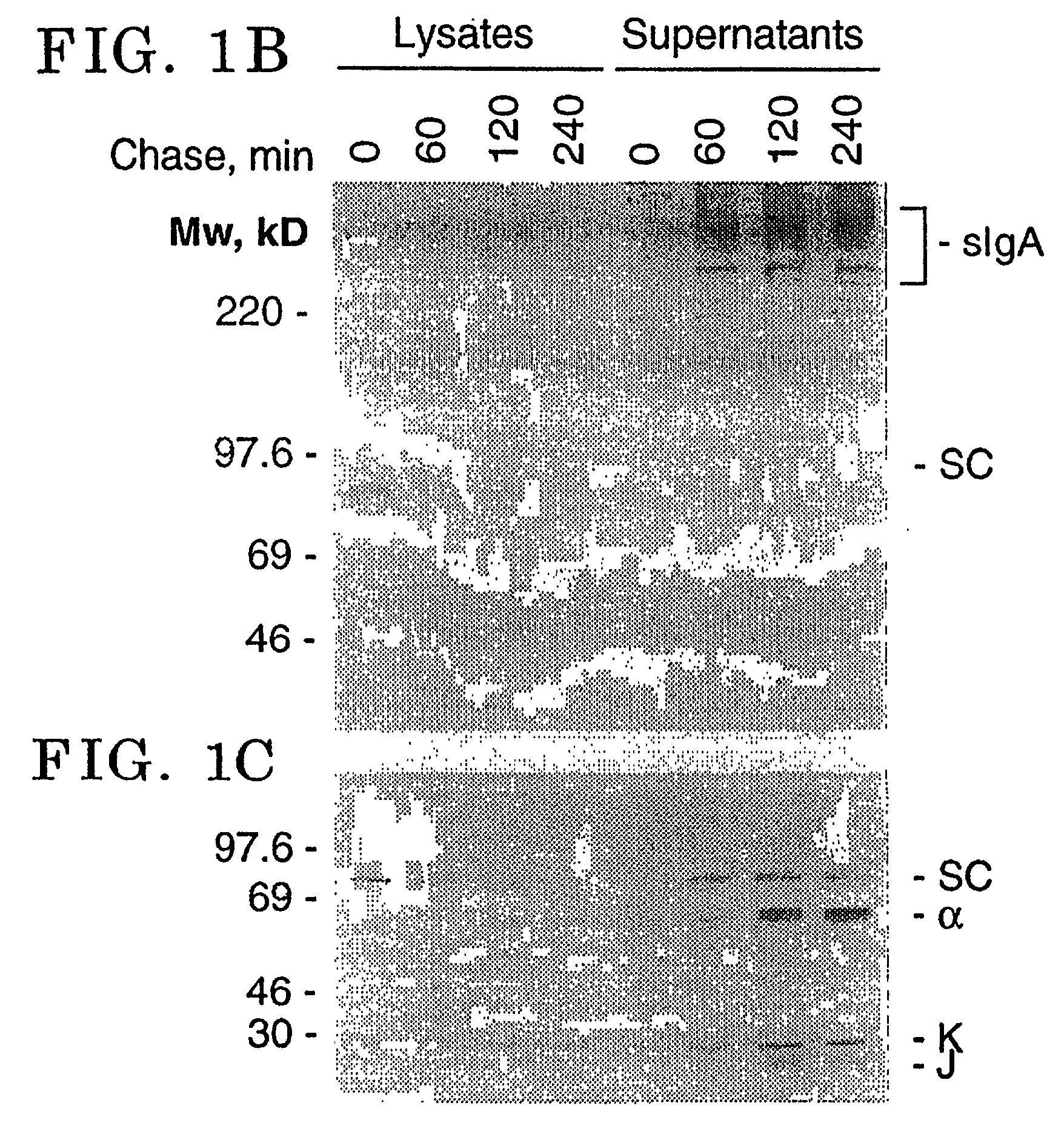
[0063] Sp2 / 0 transfectants secreting monomeric and dimeric forms of mouse-human chimeric IgA1 have been previously reported (Chintalacharuvu et al., J. Immunol. 157 3443, 1996). Cells secreting mouse-human chimeric IgA1 were transfected with the SC expression vector by electroporation. Sp2 / 0 cells were plated in 96-well tissue culture plates in presence of Histidinol. The surviving colonies were screened for SC secretion by ELISA using goat anti-.kappa. as the trapping antibodies and rabbit anti-human SC as the detecting antibody. The clone producing the highest quantity of sIgA was expanded and adapted to growth in serum free medium.
[0064] Since SC is a cleavage product of the pIgR a stop codon was introduced at the site of cleavage (FIG. 1A). Murine transfectomas secreting mouse-human chimeric IgA1 specific for the hapten dansyl (Chintalacharuvu et al., J. Immunol 157 3443, 1996) were transfe...
example 3
Analysis of Culture Supernatants
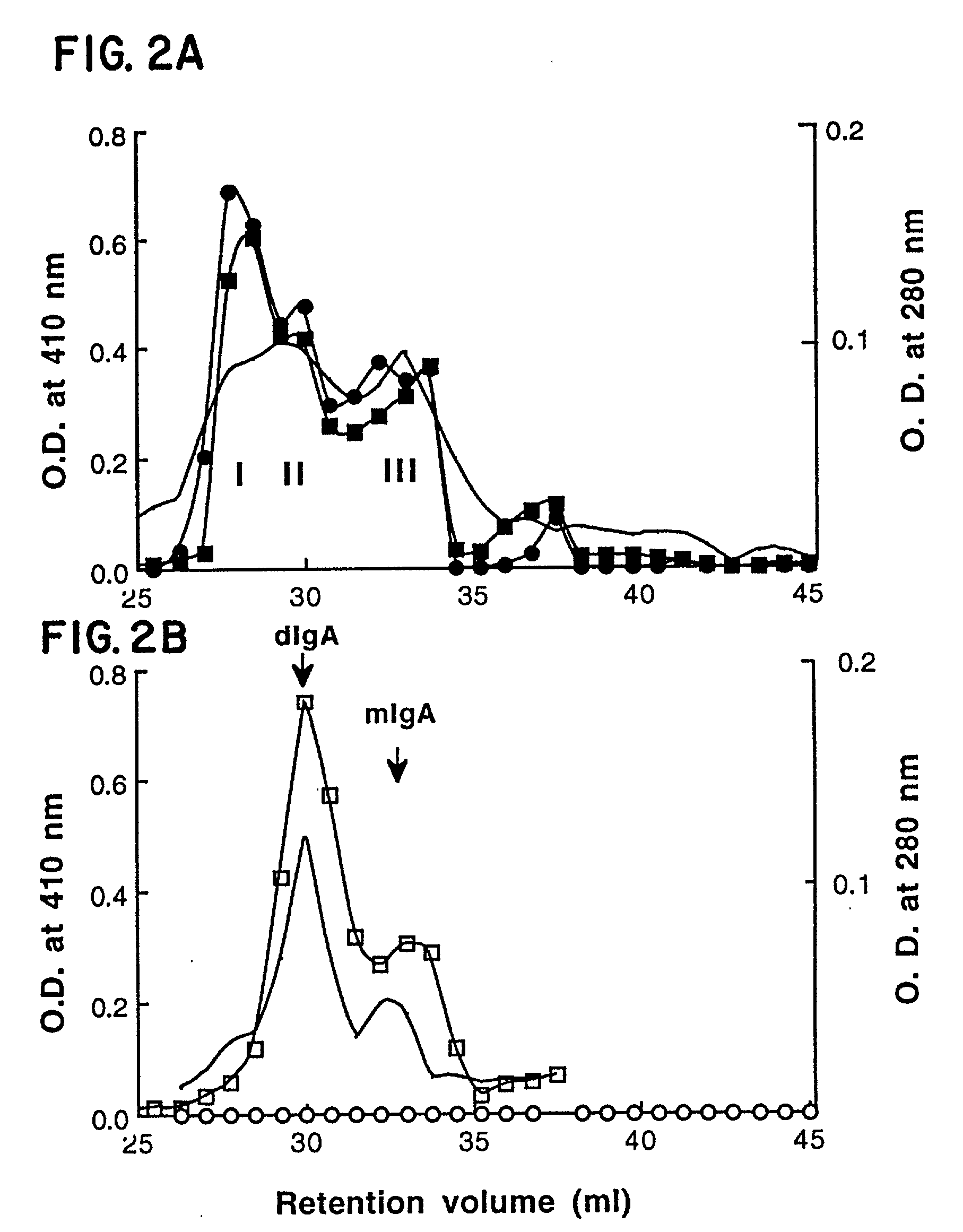
[0065] The levels of sIgA in culture supernatants were determined by ELISA as described previously (Chintalacharuvu et al., J. Immunol 157 3443, 1996). Microtiter plates coated with dansylated BSA was used to capture sIgA. Bound sIgA was detected by incubation with rabbit antiserum to human SC (Chintalacharuvu et al., J. Cell. Physiol. 148 35, 1991) diluted 1:2000 in phosphate buffered saline (PBS) containing 1% (w / v) BSA (PBS-1% BSA). Bound rabbit antibody was detected using an alkaline phosphatase conjugated goat anti-rabbit IgG (Sigma Imm. Chem.) diluted 1:10,000 in PBS-1% BSA. Color was developed by adding 5 mg / ml of disodium p-nitrophenyl phosphate (Sigma Imm. Chem.).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| retention volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com