Treating compositions with lactoferrin
a technology of lactoferrin and composition, applied in the field of treating compositions with lactoferrin, can solve the problems of limiting the production of lactoferrin from human milk, affecting the production efficiency of lactoferrin, and causing pathological conditions,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
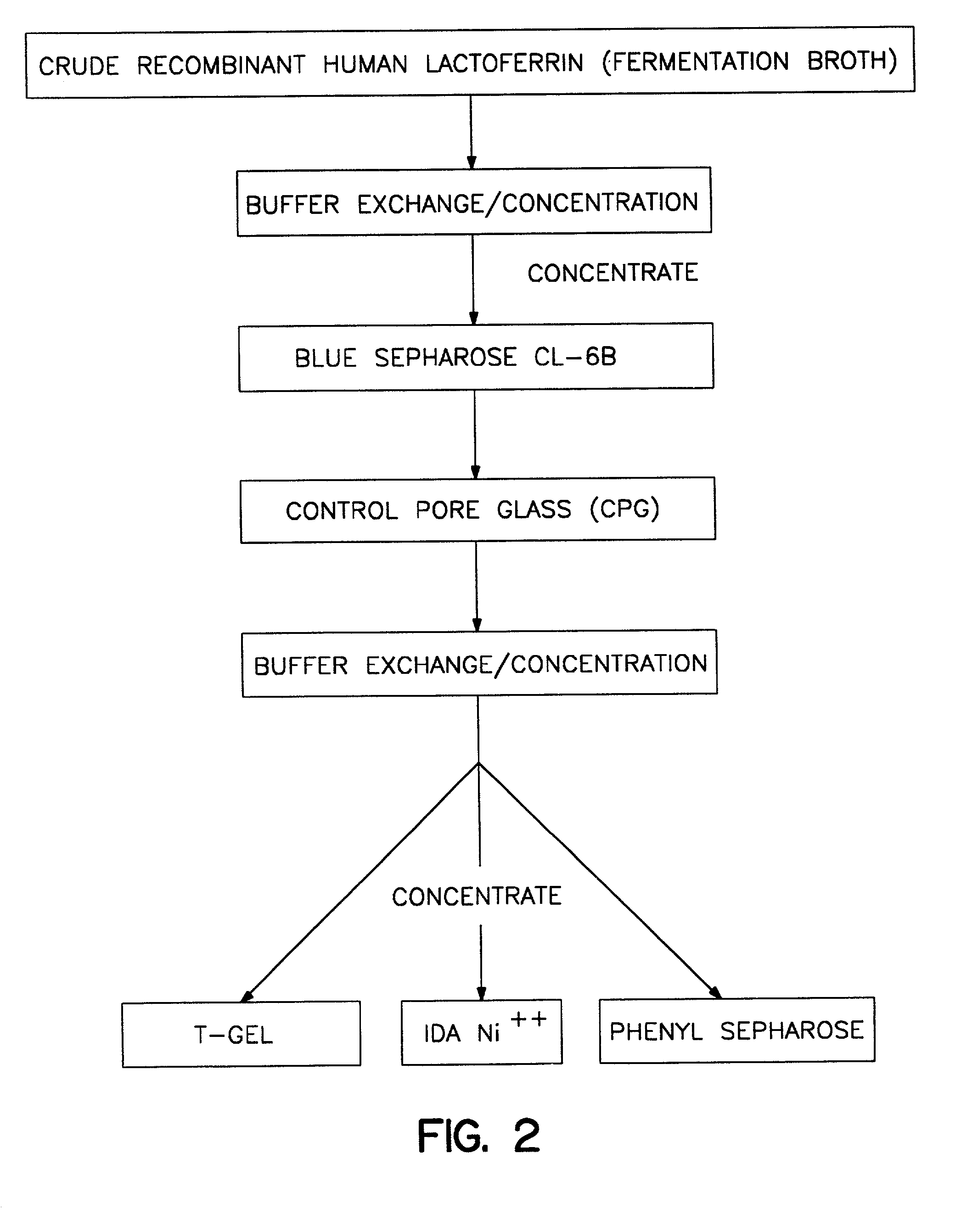
Concentration and initial Purification of Lactoferrin
[0045] One liter of the supernatant from Example 1 is adjusted to about 40C. and filtered under pressure through a polysulfone ultrafiltration membrane having a pH operating range of 1-14 on a polypropylene mesh support (PELLICON.TM. Cassette filter System assembled with Procon pump and PTGC membrane, Millipore Corporation, Milford, Mass.) to retain proteins in excess of about 10,000 molecular weight. Pressure with simultaneous circulation is applied until 900 ml of ultra filtrate is collected. A flow rate of about 100 ml per minute is maintained during the filtration process. The retained material (100 ml) is diluted with 900 ml 20 mM phosphate buffer (pH 7.4) and re-filtered, which is repeated four times (final exchange ratio=10,000). The final material retained is sterilized (0.22 micron GELMAN.TM. filter).
example 3
Purification of Lactoferrin using Affinity Chromatography
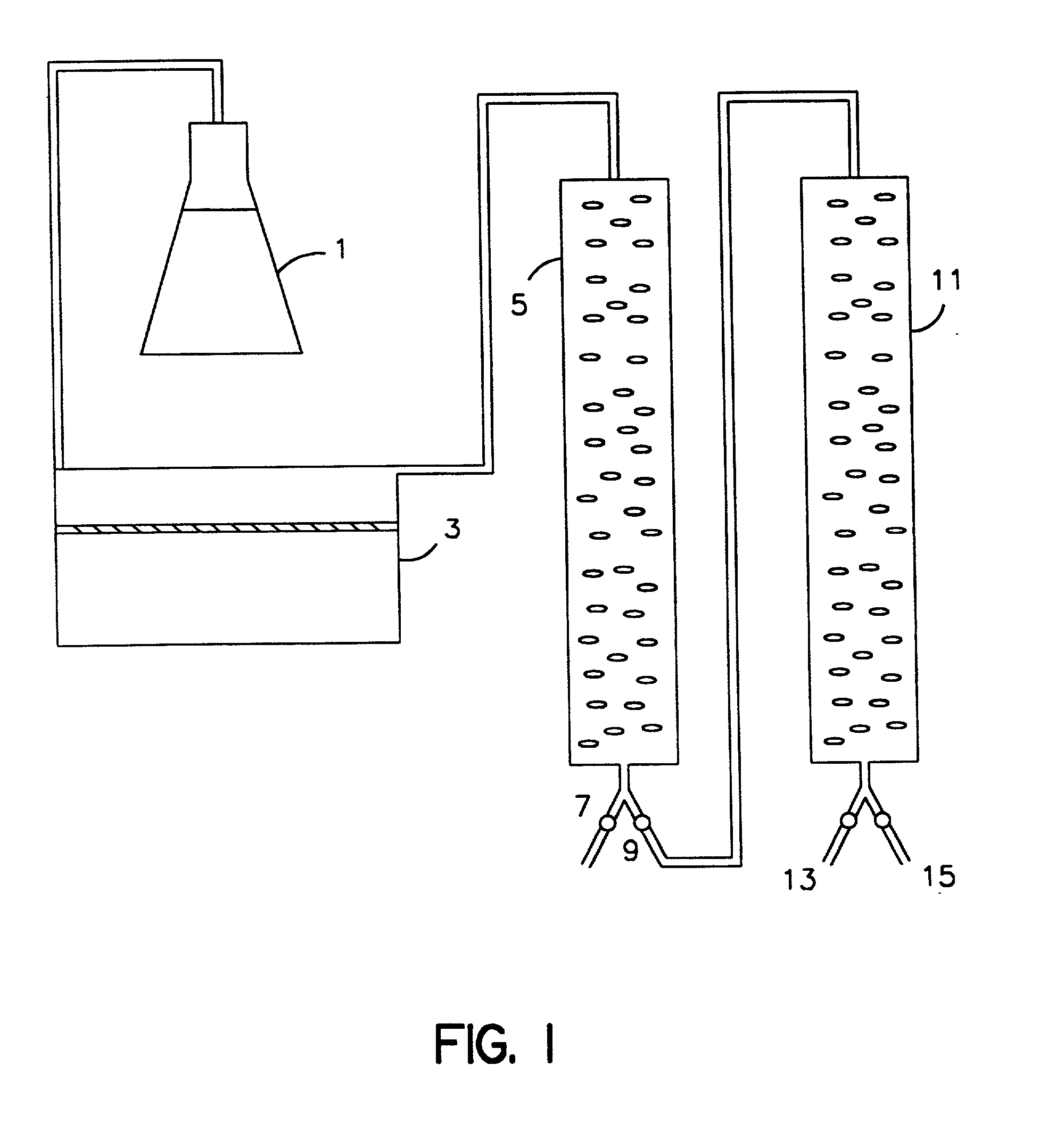
[0046] Human lactoferrin is purified using affinity chromatography in which the affinity ligand is the reactive dye Cibacron blue F3G-A. The sterilized material obtained in Example 2 is adjusted to a pH of 7.5 and a final concentration of sodium chloride of 0.5 M. This material is then applied onto a column (5 cm.times.35 cm) packed with cross-linked agarose coupled to the dye (Pharmacia Fine Chemicals, Uppsala, Sweden, BLUE SEPHAROSE.TM. CL-6B) and previously equilibrated with 50 mM N-[2-hydroxyethyl]piperazine-N'-[2-ethanesulfonic acid] (HEPES) buffer (pH 7.5) containing 0.5 M sodium chloride. Adsorption is performed at a flow rate of 1 ml / min followed by washing the column with 2 bed volumes of the same HEPES buffer. The non-adsorbed fraction is discarded and the adsorbed fraction containing lactoferrin is eluted from the column bed using 2 bed volumes of 50 mM HEPES buffer (pH 7.5) containing 1 M sodium chloride.
example 4
Purification of Lactoferrin using Control Pore Glass (CPG) Chromatography
[0047] Human lactoferrin is purified using control pore glass (CPG) chromatography. The eluate from Example 3 is applied onto a column (1.2 cm.times.10 cm) packed with CPG beads (CPG 00350, ElectroNucleonics, Fairfield, N.J.) and previously equilibrated with 50 mM HEPES buffer (pH 7.5) containing 1 M sodium chloride. Adsorption is performed at a flow rate of 1 ml / min followed by washing the column with 2 bed volumes of the same buffer. The non-adsorbed fraction is discarded, and the adsorbed fraction is eluted with 2 bed volumes of 0.25 M tetramethylammonium chloride (TMAC, pH 7.5). The eluate is filtered on a membrane capable of excluding material having a molecular weight greater than 10,000 daltons (AMICON.TM. YM 10). The filtered material is then sterilized (0.22 micron GELMAN.TM. filter) and frozen at -200C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com