Electrochemical test strip for reducing the effect of direct interference current
a technology of direct interference current and test strip, which is applied in the field of electrochemical strips, can solve the problems of unsatisfactory oxidation current, reduce the oxidation current, and the technique is not always successful, and achieve the effect of more accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
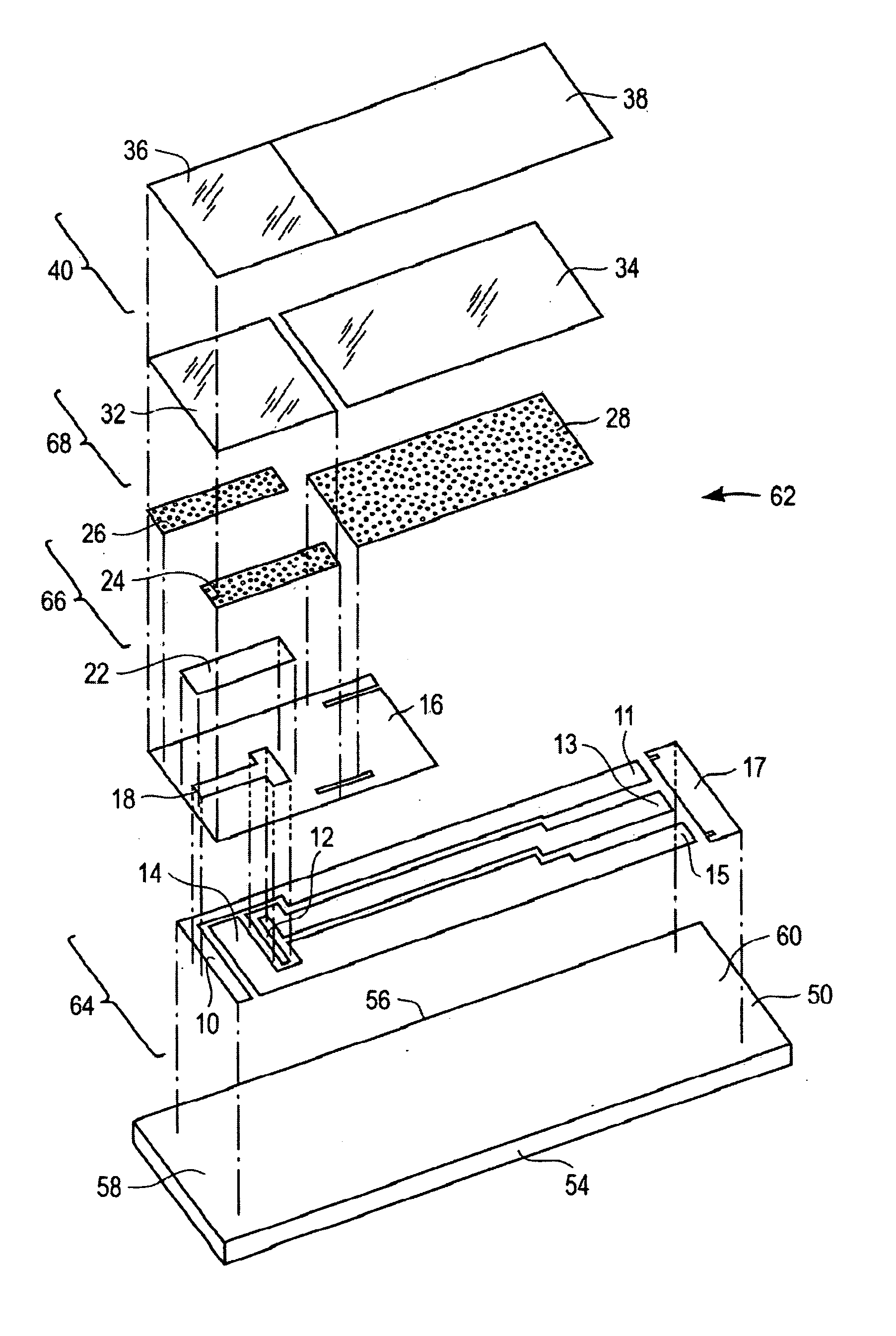
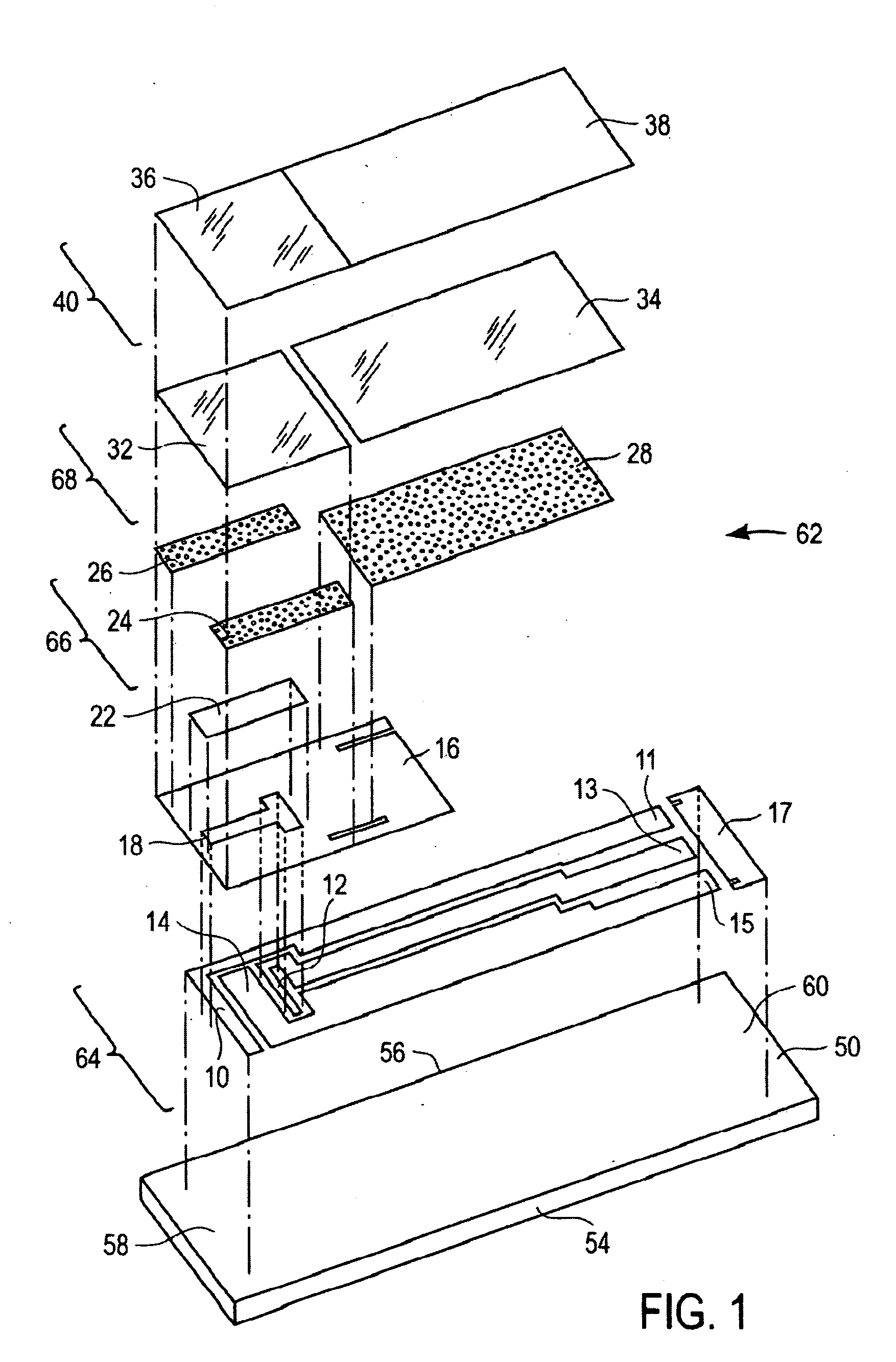
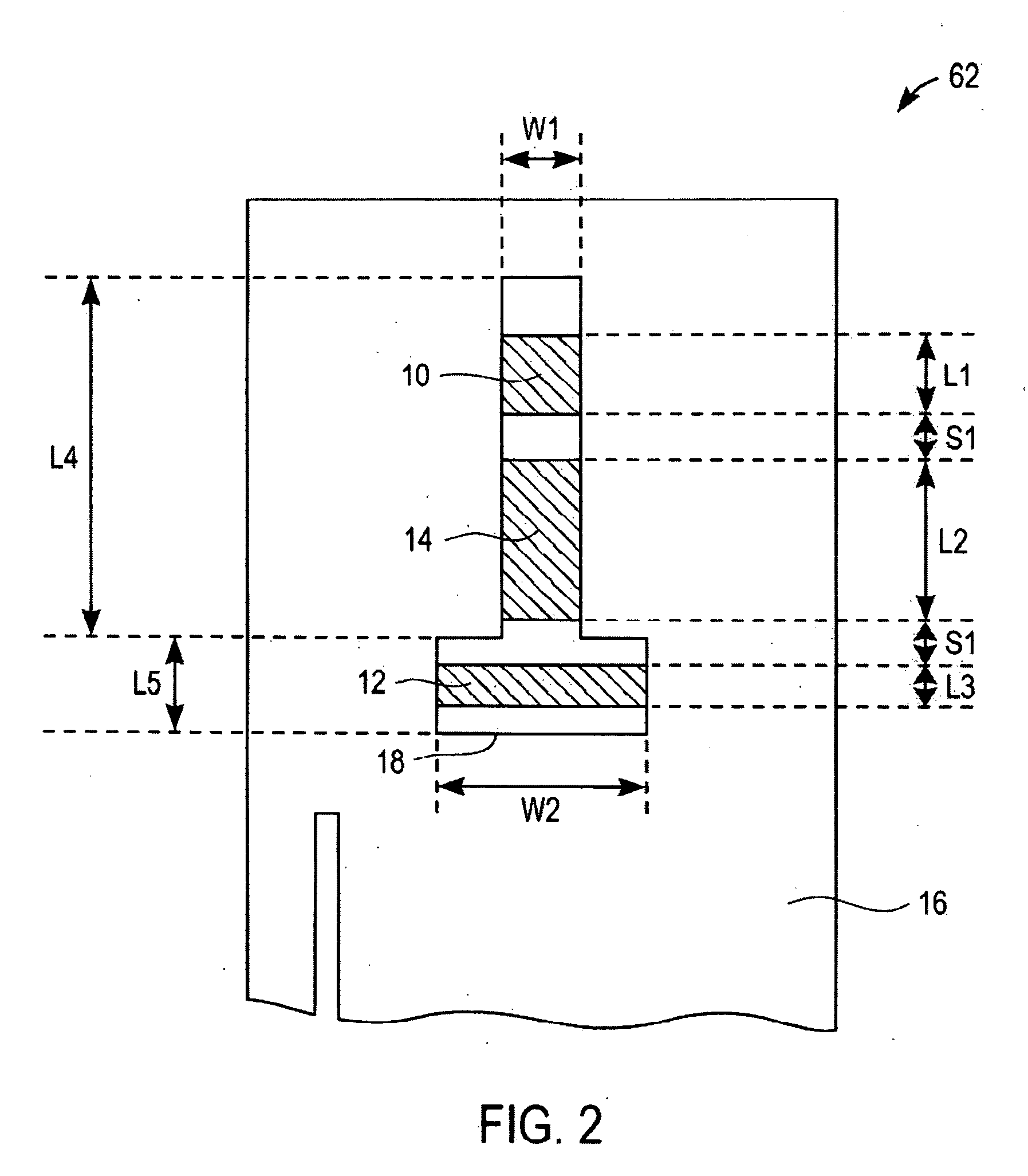
[0065] Test strips were prepared according to the first embodiment of the present invention as illustrated in FIG. 1 to 3. These test strips were tested in blood having various concentrations of interferents. To test these strips, they were electrically connected to a potentiostat which has the means to apply a constant potential of 0.4 volts between the first working electrode and the reference electrode; and the second working electrode and the reference electrode. A sample of blood is applied to the sample inlet allowing the blood to wick into the sample receiving chamber and to wet first working electrode, second working electrode, and reference electrode. The reagent layer becomes hydrated with blood and then generates ferrocyanide which may be proportional to the amount of glucose and / or interferent concentration present in the sample. After about 5 seconds from the sample application to the test strip, an oxidation of ferrocyanide is measured as a current for both the first a...
example 2
[0068] To show that the method of correcting the current for interferents applies to a wide variety of interferents, strips built according to the embodiment of FIG. 1 were also tested with acetaminophen and gentisic acid at various concentration levels, in addition to uric acid. For purposes of quantitating the magnitude of this effect, a change in glucose output of greater than 10% (for glucose level >70 mg / dL) or 7 mg / dL (for glucose level <=70 mg / dL) was defined as a significant interference. Table 1 shows that the uncorrected current at the first working electrode shows a significant interferent effect at a lower interferent concentration than strips tested with a corrected current response using Equation 7a. This shows that the method of correcting the current output of the first working electrode using Equation 7a is effective in correcting for interferences. Table 1 shows that the current correction in Equation 7a is effective for interferences with respect to acetaminophen,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com