Very large scale immobilized polymer synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Contents
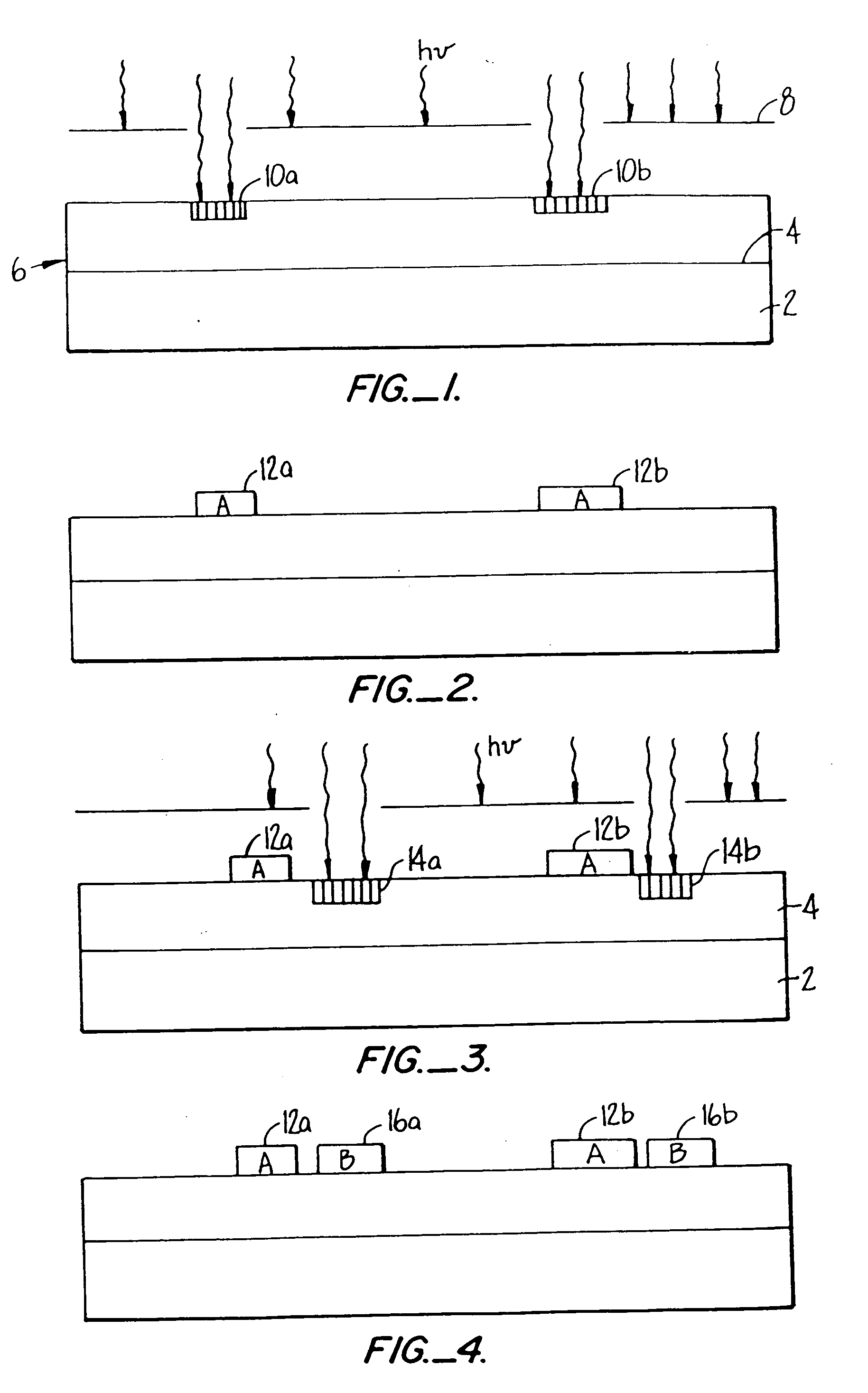
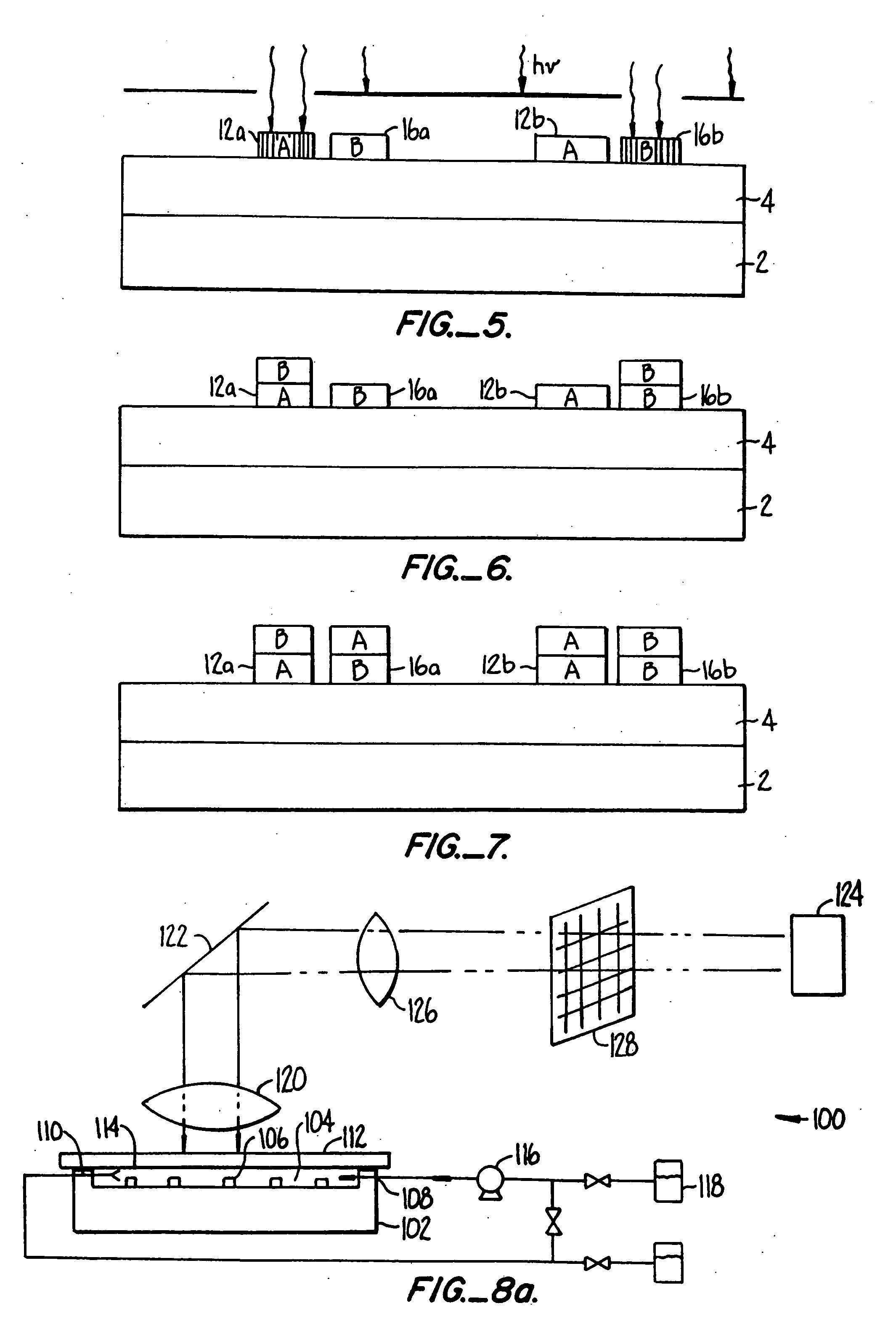
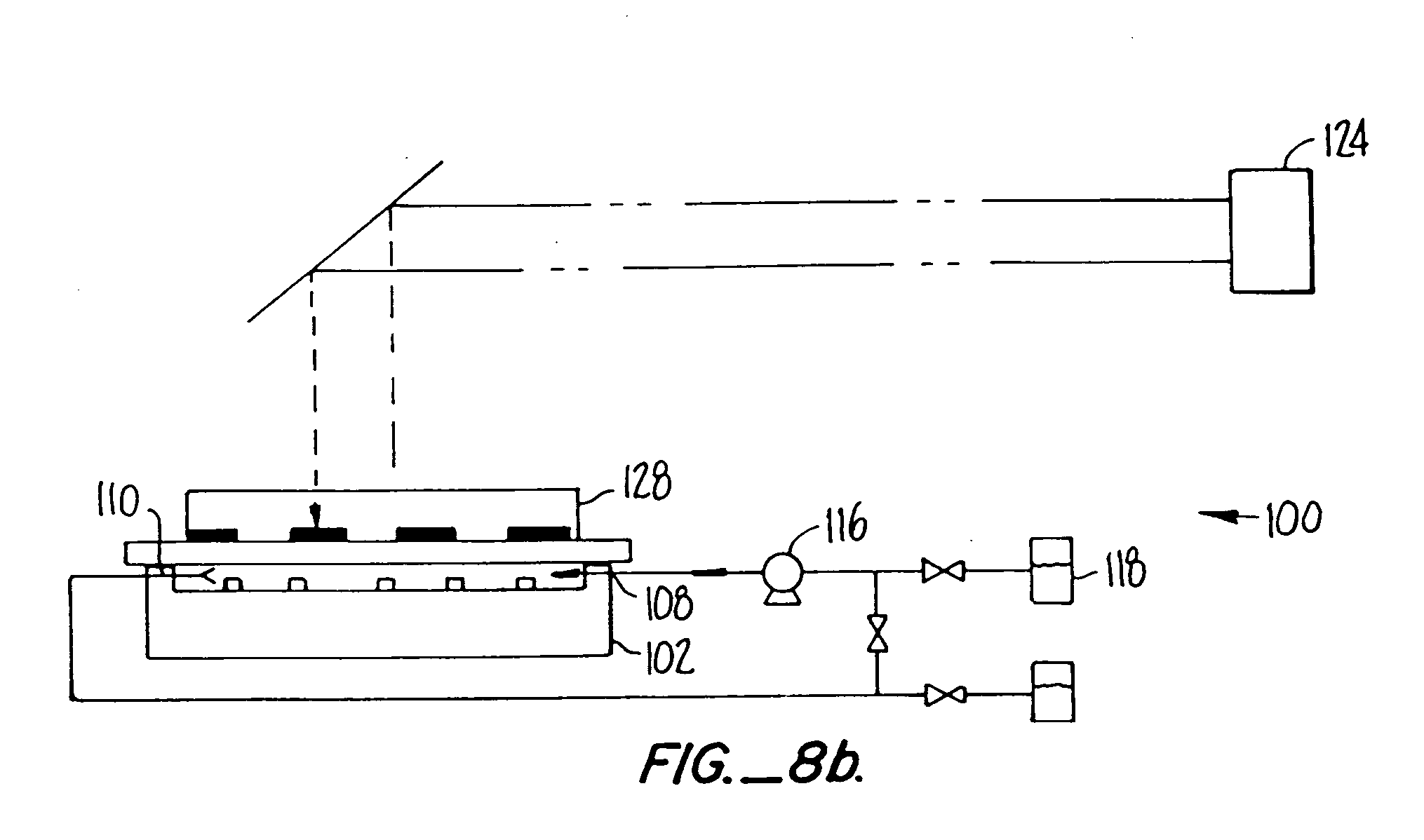
[0041] I. Glossary [0042] II. General [0043] III. Polymer Synthesis [0044] IV. Details of One Embodiment of a Reactor System [0045] V. Details of One Embodiment of a Fluorescent Detection Device [0046] VI. Determination of Relative Binding Strength of Receptors [0047] VII. Examples [0048] A. Slide Preparation [0049] B. Synthesis of Eight Trimers of “A” and “B”[0050] C. Synthesis of a Dimer of an Aminopropyl Group and a Fluorescent Group [0051] D. Demonstration of Signal Capability [0052] E. Determination of the Number of Molecules Per Unit Area [0053] F. Removal of NVOC and Attachment of a Fluorescent Marker [0054] G. Use of a Mask in Removal of NVOC [0055] H. Attachment of YGGFL and Subsequent Exposure to Herz Antibody and Goat Antimouse [0056] I. Monomer-by-Monomer Formation of YGGFL and Subsequent Exposure to Labeled Antibody [0057] J. Monomer-by-Monomer Synthesis of YGGFL and PGGFL [0058] K. Monomer-by Monomer Synthesis of YGGFL and YPGGFL [0059] L. Synthesis of an Arra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com