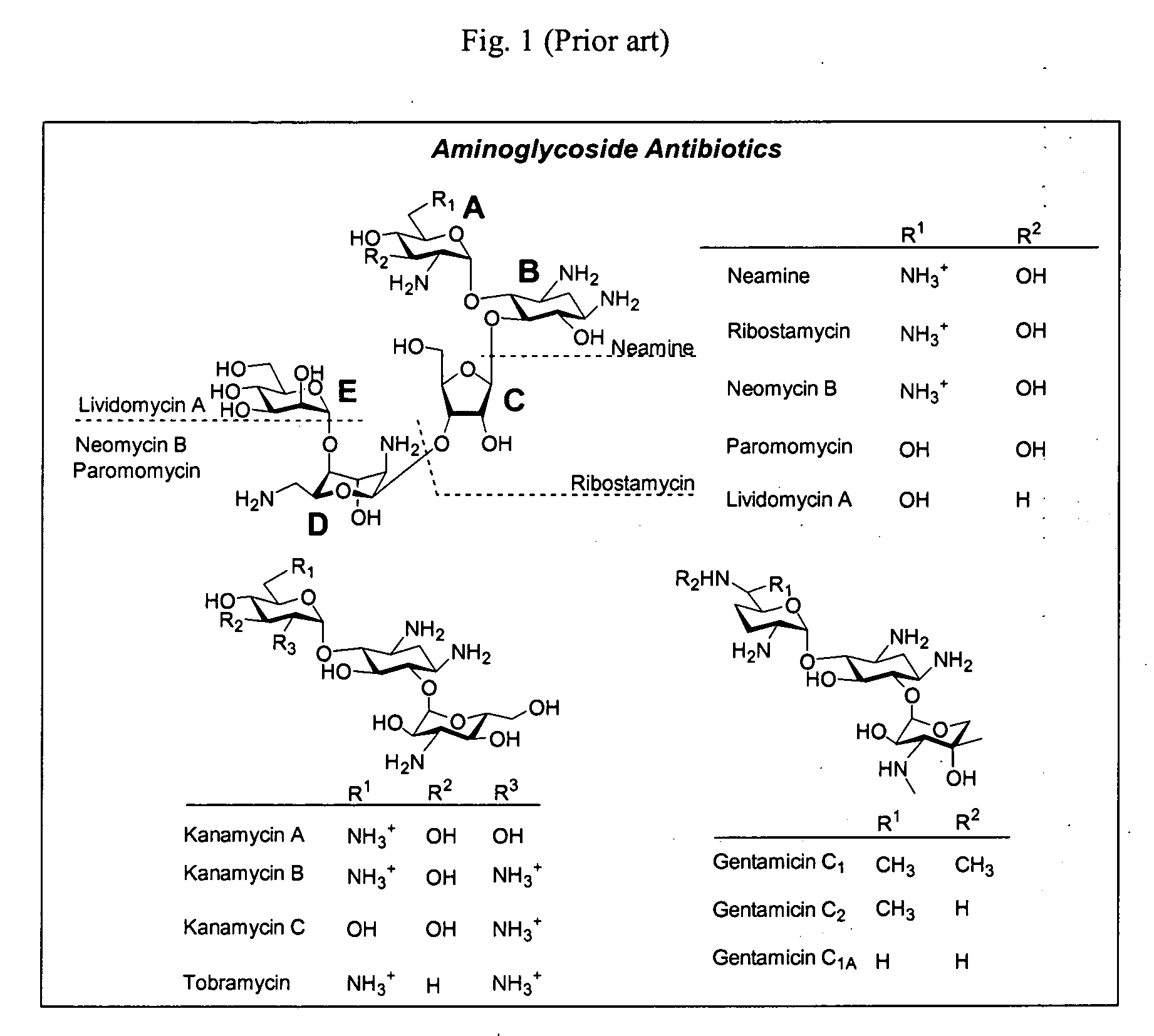
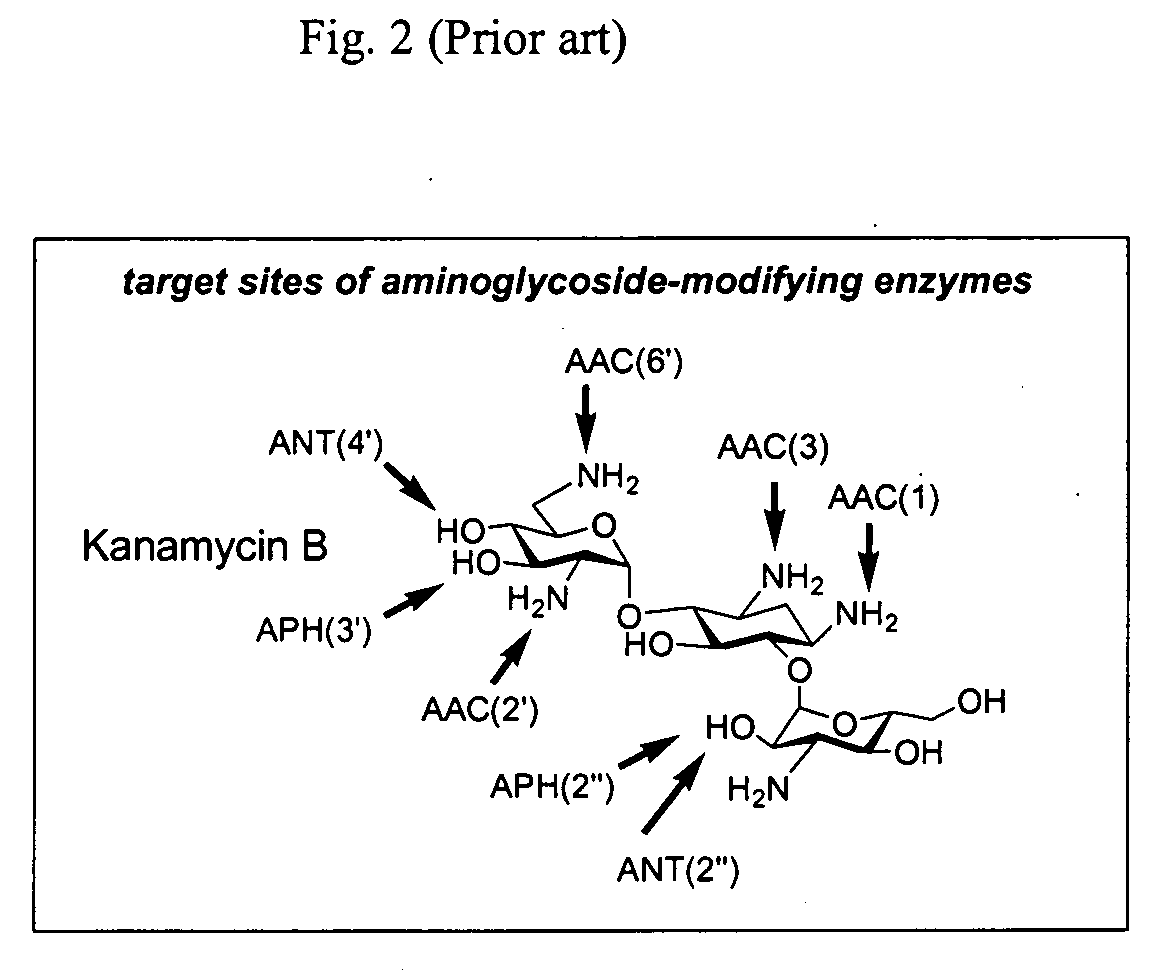
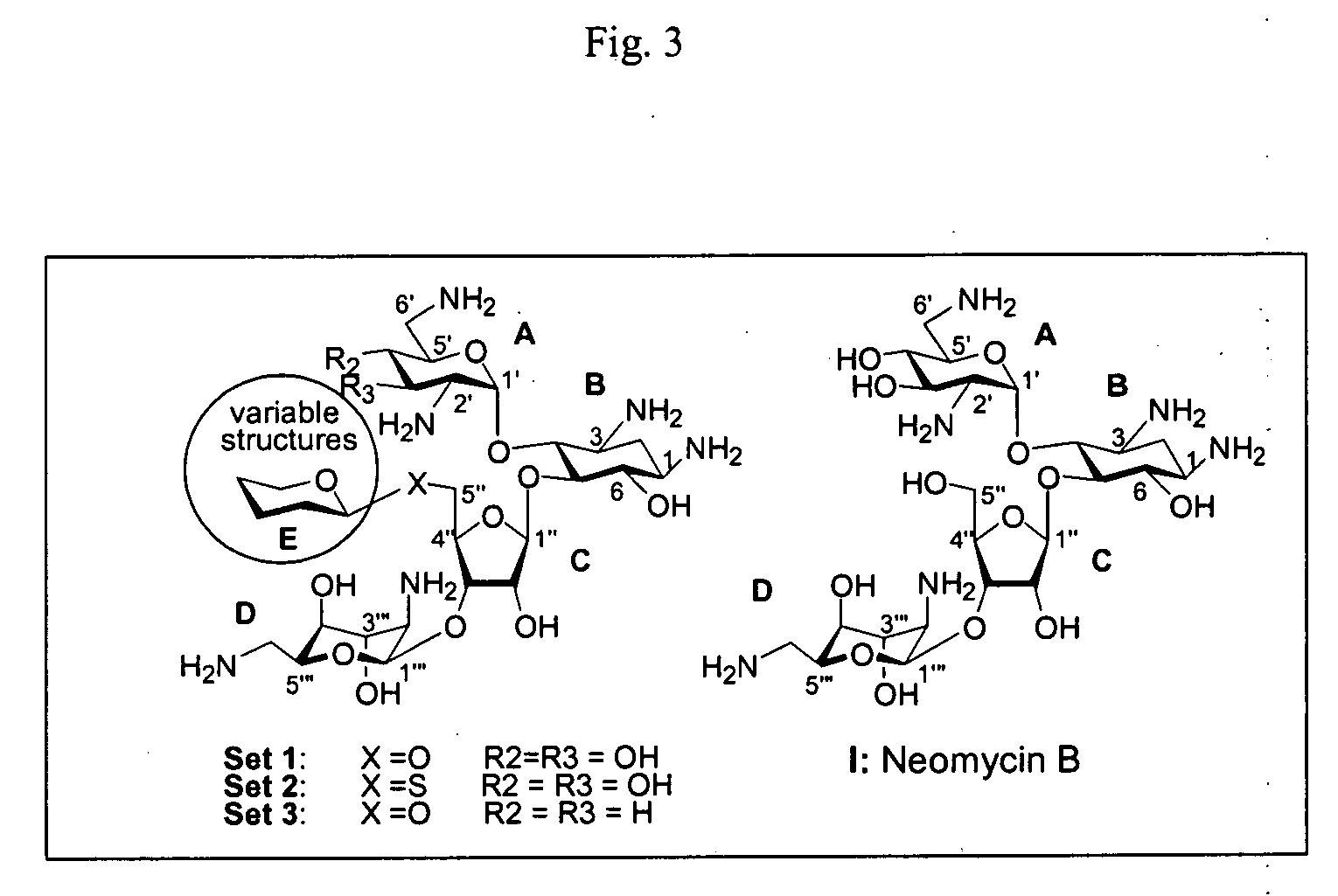
Bifunctional antibiotics for targeting rRNA and resistance-causing enzymes and for inhibition of anthrax lethal factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
General Synthesis of the Compounds of the Present Invention and Syntheses of Specific Exemplary Intermediates
[0130] The strategy for the construction of all three sets of compounds in FIG. 3 featured the use of a common acceptor for each set (acceptors 1-3 in FIG. 4), to which the monosaccharide donors were connected, followed by a two-step deprotection to yield the target C5″-branched derivatives. The protecting groups used in this study served admirably in terms of ease of attachment and removal and survivability under the reaction conditions, whereas the thioglycoside-NIS (31) and trichloroacetimidate-BF3 (32) glycosidation methods proved to be both rapid and efficient.
[0131] This Example describes the overall synthetic procedure with optional variations; the following Examples include specific non-limiting examples of the synthetic process as it was performed for the present invention.
[0132] The neomycin acceptor 1 is readily accessible from the commercial neomycin B (49). Th...
example 2
Selection of Structures for Compounds of the Present Invention
[0168] The previous Example related to a general scheme which may optionally be used for any compound according to the present invention, as well as optionally for generating a library of compounds according to the present invention. This Example describes the selection of some non-limiting, illustrative structures for compounds according to the present invention.
[0169] One important aspect of the present invention is the use of functional aminoglycosides to solve the problem of cytotoxicity. Without wishing to be limited by a single hypothesis, these structures were selected to ameliorate this problem. One of the major drawbacks of aminoglycosides is their relatively high toxicity. Neomycin B is the most toxic of aminoglycosides, yet it is primarily used for topical infections. It is highly nephrotoxic and ototoxic and is by far the most potent in the area of neuromuscular blockage. Aminoglycosides are nephrotoxic beca...
example 3
Specific Synthesis of Selected Compounds of the Present Invention
[0174] Example 1 included a general synthetic scheme which may optionally be used for any compound according to the present invention, as well as optionally for generating a library of compounds according to the present invention. This Example provides an illustrative, non-limiting synthetic process that was performed for selected compounds according to the present invention.
[0175] As shown in FIGS. 10-12, a compound was prepared according to a synthetic scheme which started with neomycin B being converted to a general acceptor, as described with regard to Example 1. FIGS. 10 and 11 show the syntheses of the monosaccharide donors. Neomycin B (Compound I) is shown in FIG. 12 after being converted to an acceptor 1 to which the monosaccharide donors of FIG. 7 can be coupled.
[0176] The protecting groups used in this study served admirably in terms of the ease of attachment and removal and survivability under the reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com