Methods for stably incorporating substances within dry, foamed glass matrices and compositions obtained thereby
a technology of foamed glass and compositions, applied in the direction of pharmaceutical delivery mechanisms, pill delivery, heterocyclic compound active ingredients, etc., can solve the problems of large loss, loss of whole batches at production level, and many freeze-dried substances are still unstable at ambient temperatures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of Vacuum Pressure and External Temperature on Primary Drying Times
[0066] 2 ml aliquots of 10% (w / v) trehalose in deionized distilled water were placed in 10 ml Wheaton pharmaceutical vials and dried in an FTS drier at various vacuum pressures and shelf temperature settings. The sample temperatures and the time taken to remove approximately 90% of the water (i.e. primary drying to give a syrup) were determined. The results are shown in Table 1.
example 2
Formation of FGMs
2a. Formation from an Aqueous Solution of Glass Matrix-Forming Material
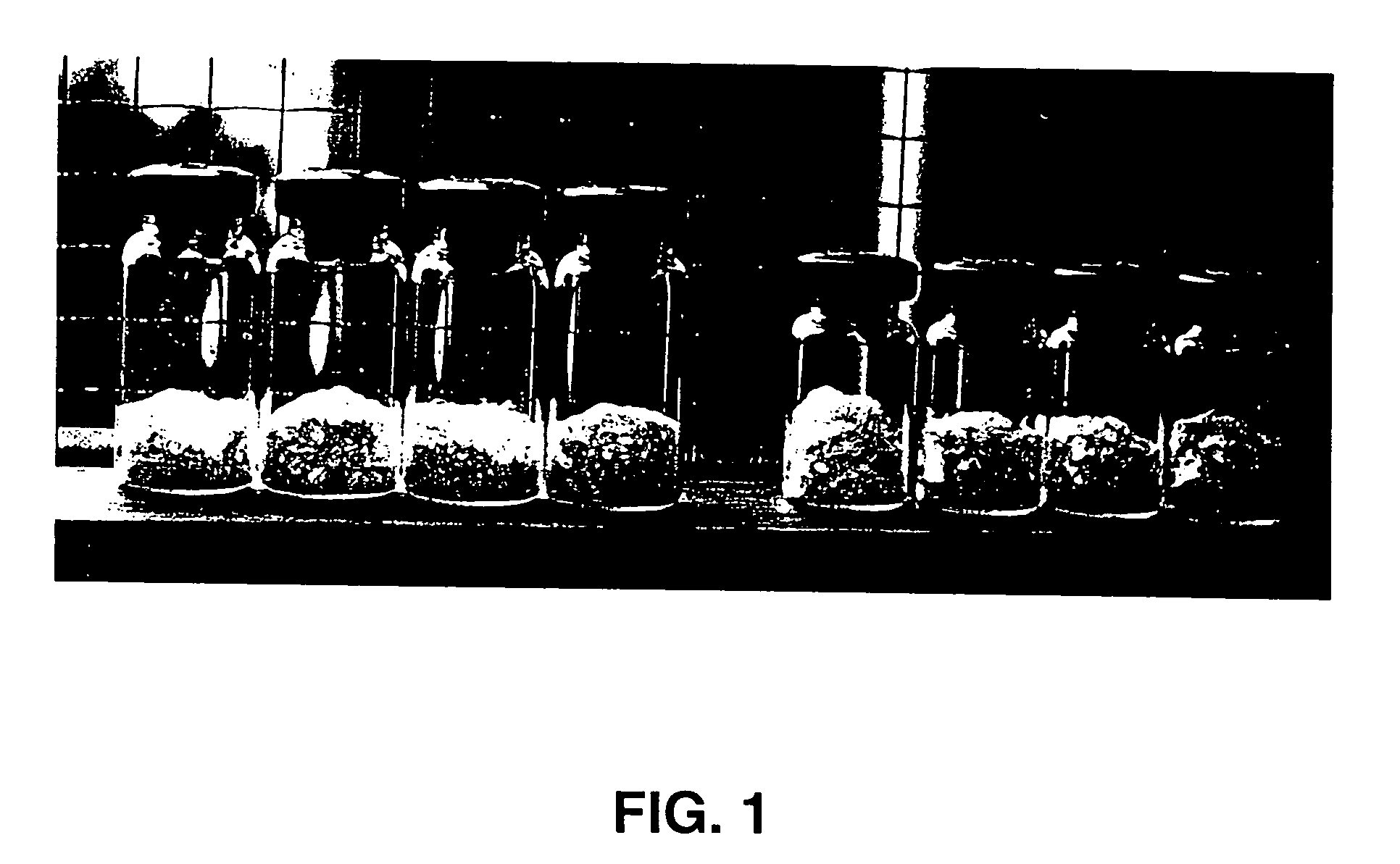
[0067] Aliquots of 250, 410 μl and 500 μl of a 50% (w / v) solution of trehalose in 3 ml, 5 ml and 10 ml pharmaceutical vials respectively, were dried in an FTS drier for 16 hrs. The shelf temperature was maintained at 25° C. throughout the run and the vacuum pressure dropped to 0.03 Torr / mm Hg within the first 15 mins of the run and maintained at 0.03 Torr / mm Hg throughout the run. The FGMs formed are shown in FIG. 1A. The foam-like appearance is due to the instantaneous drying of the bubbles that form during the boiling step.
2b. Formation From an Aqueous Solution of Glass Matrix-Forming Materials Incorporating Active in Solution
[0068] Recombinant Hepatitis B Surface Antigen in 20% (w / v) trehalose±0.5% (w / v) Byco A in PBS was dried in 300 μl volumes in 3 ml pharmaceutical vials. The FTS drying protocol involved a pressure of 0.03 Torr / mm Hg with shelf temperature maintained at 40° C. throughou...
example 3
Effect of Vacuum Pressure / Shelf Temperature on FGM Formation
3a. Formation From Solution of Glass Matrix-Forming Material Plus Additive
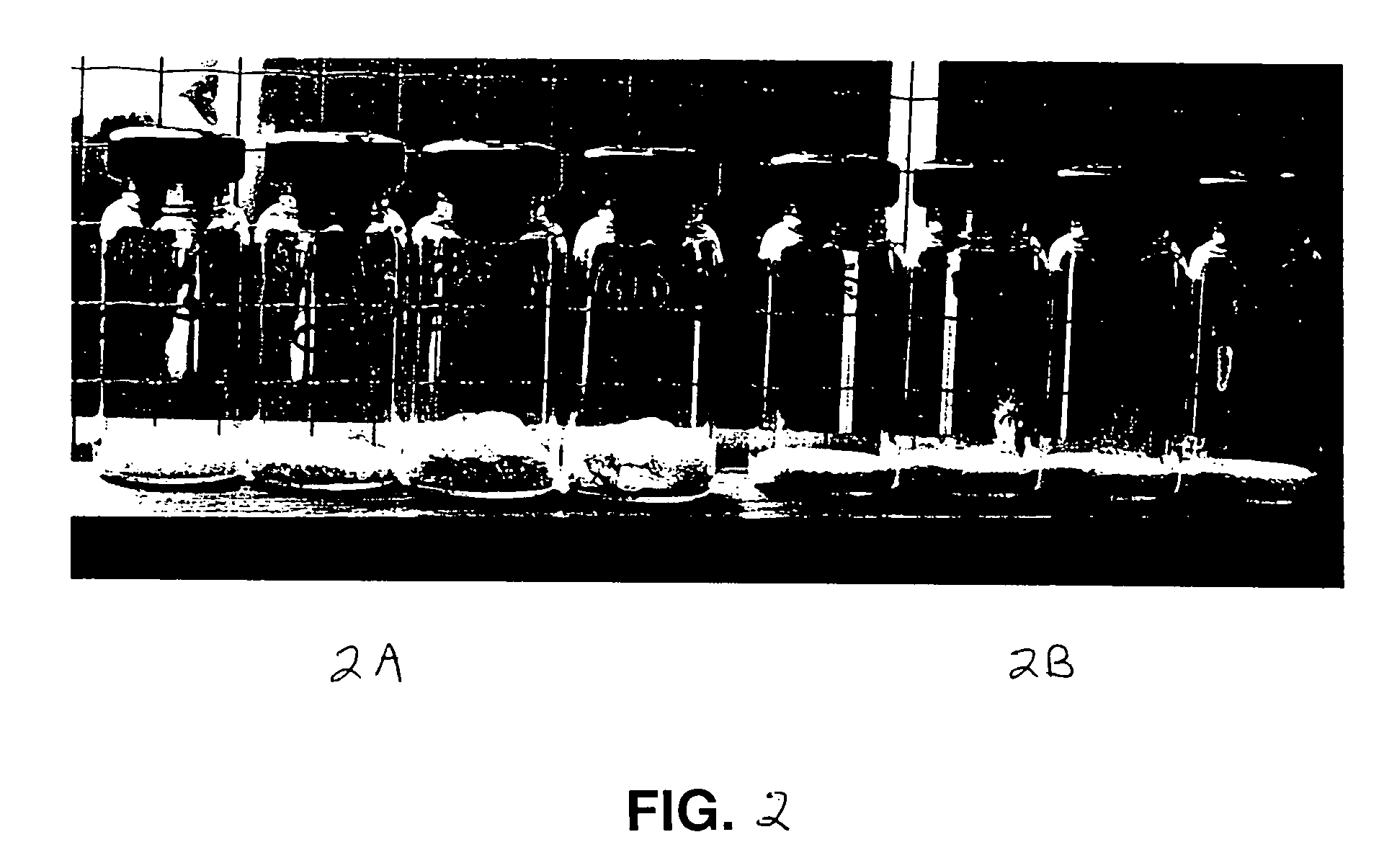
[0078] Aliquots of 1 ml or 500 μl of 25% (w / v) trehalose containing either 0.25 or 0.5 M ammonium bicarbonate, were dried in 10 ml pharmaceutical vials in the FTS drier. The 1 ml samples were dried at a constant vacuum pressure of 0.03 Torr / mm Hg for 14 hrs, with shelf temperature initially 25° C., raised to 45° C. after the first 2 hours (i.e., syrup formed). The 500 μl samples were dried at a constant shelf temperature of 25° C. and a constant vacuum-pressure of 0.01 Torr / mm Hg for 14 hr. The FGMs formed (FIG. 2A) occupied larger volumes than identical samples processed by freeze-drying (FIG. 2B).
3b. Formation From Solution of Glass Matrix-Forming Material Incorporating an Active
[0079] 300 μl aliquots of a solution of 43.4 mg / ml trehalose containing 66 mg / ml of an antimicrobial peptide was dried in 10 ml polypropylene tubes (10 mm diameter) in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Bioactive | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com