Methods, compositions, and kits for protein crystallization
a protein and composition technology, applied in the direction of fluid pressure measurement, liquid/fluent solid measurement, peptides, etc., can solve the problems of crystal degradation or even loss, inexact and cumbersome crystallization of proteins,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
IPG Isoelectric Focusing Crystallization of Soybean Trypsin Inhibitor
[0144] Soybean trypsin inhibitor was purchased from Sigma-Aldrich and dissolved in deionized water to a concentration of 10 mg / mL. An aliquot of 150 μL of this solution was used to rehydrate a pH 4.5 to pH 5.5. ZOOM® Strip (Invitrogen Corp., Carlsbad, Calif.) in a ZOOM® IPGRunner cassette (Invitrogen Corp., Carlsbad, Calif.) for 2 hours.
[0145] The ZOOM® IPGRunner cassette was thereafter handled essentially according to manufacturer's instructions (including removal of well-forming members, application of wicks, and contact to an IPGRunner buffer core within an electrophoresis tank, all from Invitrogen Corp., Carlsbad, Calif.) and voltage applied as follows: 175V for 15 min, followed by a ramp from 175 to 2000 V over the course of one hour, and then 2000V for 2 hours 15 minutes.
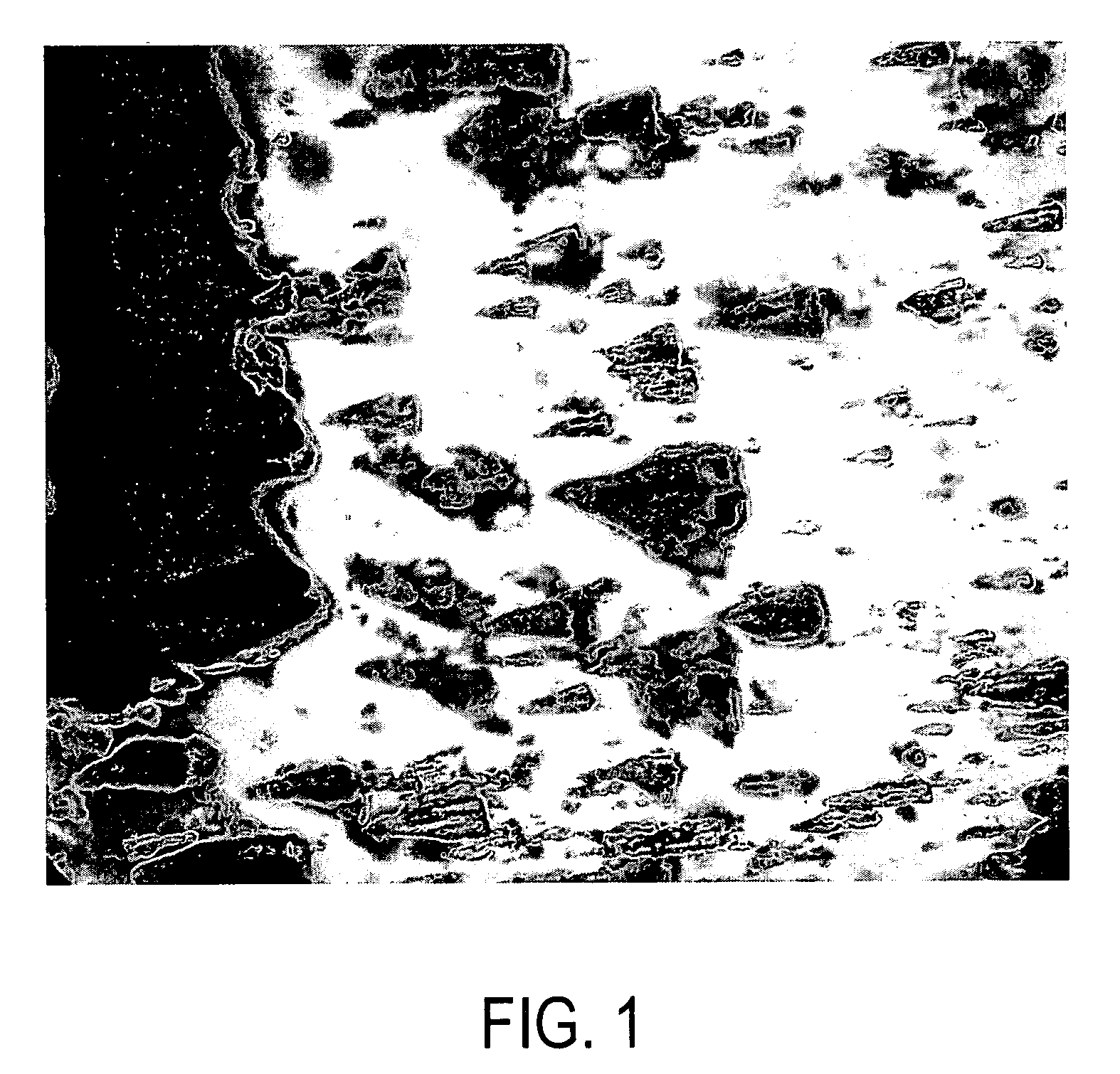
[0146] Strips were removed from the cassette and examined microscopically. Crystals of soybean trypsin inhibitor were observed in the gel...
example 2
IPG Isoelectric Focusing Preparation of Seed Crystals
[0147] Lysozyme was purchased from Sigma-Aldrich and dissolved in deionized water at a concentration of 3 mg / mL. An aliquot of 150 microliters was used to rehydrate a pH 9-12 IPG strip (prepared in-house) within an Invitrogen ZOOM® IPGRunner cassette for 1 hour.
[0148] The ZOOM® IPGRunner cassette was thereafter handled essentially according to manufacturer's instructions (including removal of well-forming members, application of wicks, and contact to an IPGRunner buffer core within an electrophoresis tank, all from Ihvitrogen Corp., Carlsbad, Calif.) and voltage applied using the following step voltage protocol: 175 volts for 15 minutes and 500 volts for 2 hours.
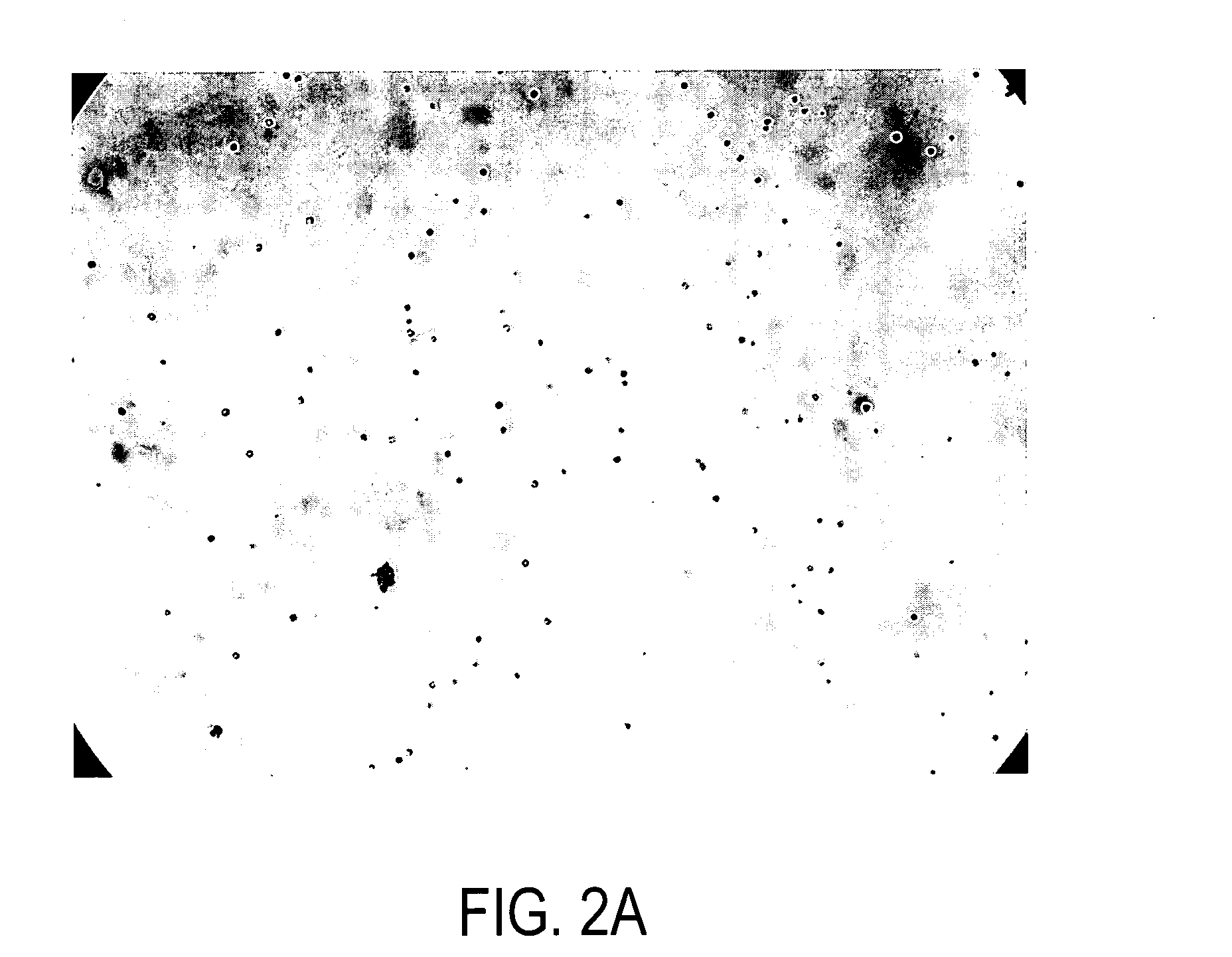
[0149] The strip was removed and examined microscopically. Micro-crystals of lysozyme were observed in the gel strip, as shown in FIG. 2A.
[0150] A section of the strip containing the micro-crystals was placed into a test tube containing a 22 mg / ml solution of lysozyme;...
example 3
IPG IEF Crystallization After Buffer Exchange
[0152] Cytochrome P450 3A4 in a buffer containing 20 mM K2PO4, 0.2 mM EDTA, 1 mM DTT, and 20% glycerol (PanVera Division of Invitrogen Corp.) was subjected to solution exchange into 0.3 mM CHAPS / 0.3 mM octyl-β-D-1-thioglucopyranoside (OTGP) using a Centricon (YM-10) 10 kDa cutoff centrifuge tube from Millipore (Billerica, Mass.).
[0153] A pH 3-10 non-linear ZOOM® Strip (Invitrogen Corp., Carlsbad, Calif.) was rehydrated in the ZOOM® IPGRunner cassette for 1 hour with a volume of 150 μL of 0.1 mg / mL of cytochrome P450 3A4 in the CHAPS / OTGP solution. Electrode wicks were wetted with 0.3 mM CHAPS / 0.3 mM OTGP and applied to the cassette, essentially as per manufacturer's instructions.
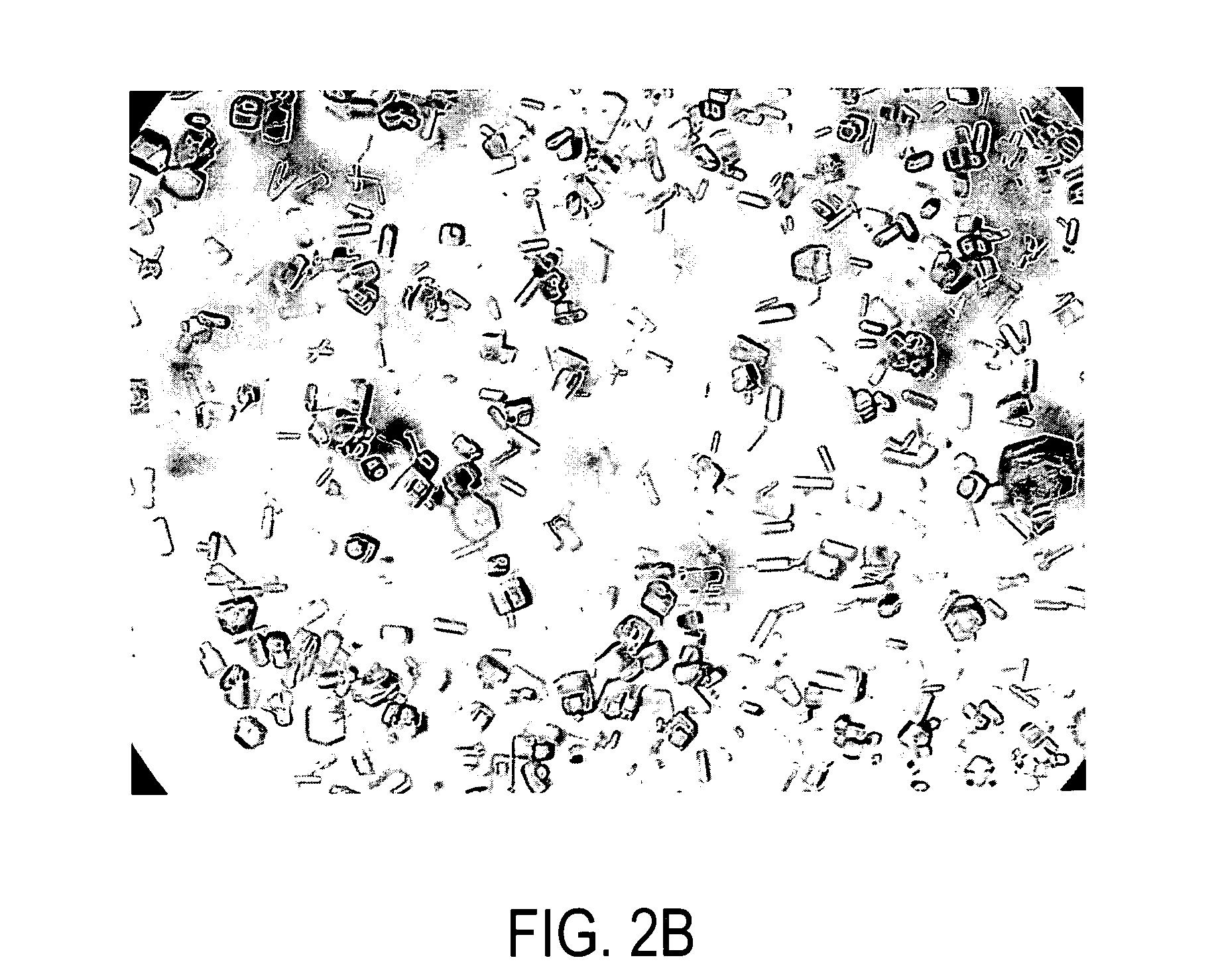
[0154] The cassette was placed in the IPGRunner apparatus and run with an applied voltage of 200 volts for 1 hour. The strips were removed and examined microscopically. Crystals observed in the gel strip were mounted in thin-walled glass capillary tubes (0.7 mm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Force | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com