Methods and compositions for impairing multiplication of HIV-1
a technology of compositions and compositions, applied in the field of methods and compositions for impairing the multiplication of hiv1, can solve the problems of nullifying the proposed mechanism of action for therapeutic benefit in hiv infection, rendering the cells susceptible to infection by the virus, etc., to reduce the viral multiplication, impair the multiplication of the virus, and reduce the viral level of hiv-1.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immunological Studies on Minimal Tat Protein Amino Acid Sequences Necessary for Binding to Antibody for Primate-Recognized Epitope I in HIV-1 Tat Protein, Sequence Variations, and Immunological Cross-Reactivities of Antiserums to these Sequences
[0136] A. Synthetic Peptide and Conjugates
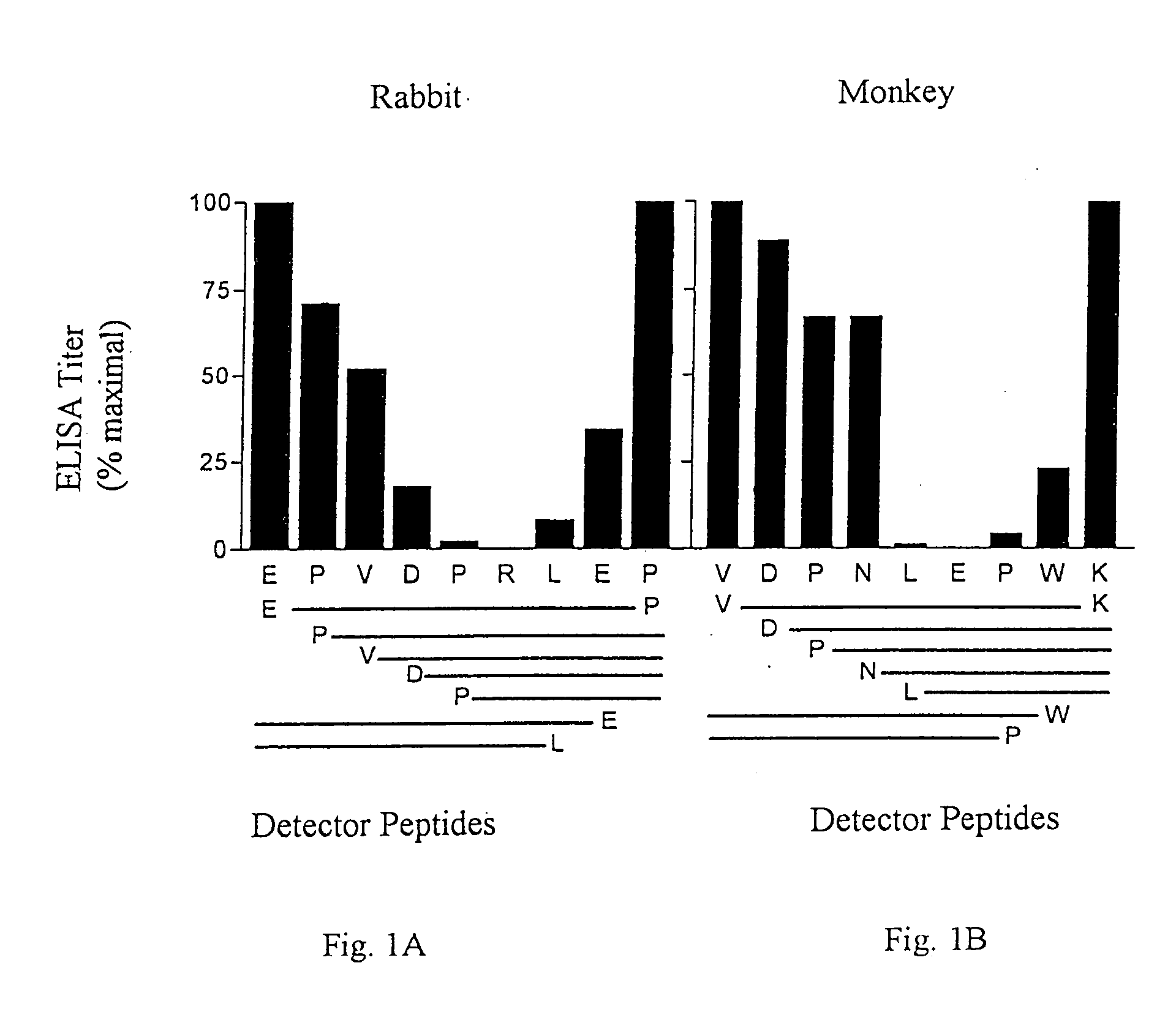
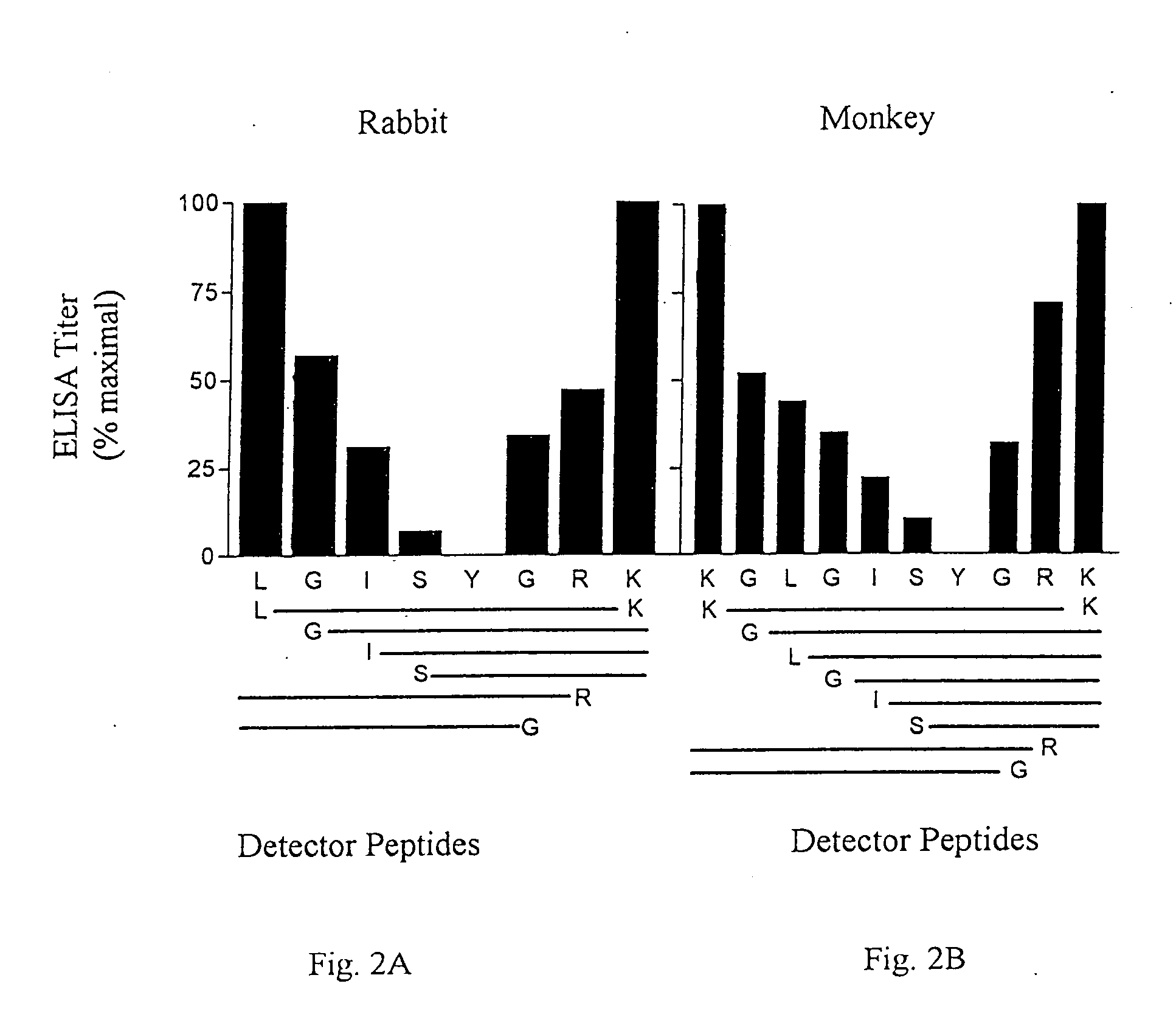
[0137] The synthetic peptides were synthesized by solid phase synthesis on derivatized polyethylene supports (R. M. Valerio et al, Int. J. Peptide Res., 44:158-165 (1994)). Immunizing peptides were synthesized with an amino terminal Cys being incorporated to facilitate coupling to a carrier protein and an amidated C-terminus. Detector peptides were synthesized with an amino terminal biotin-Ser-Gly-Ser-Gly- sequence (SEQ ID NO: 27) and a free acid function at the C-terminus for use in ELISA assays for detection of reactivity and cross-reactivity. Immunizing peptides, covalently conjugated to diphtheria toxoid (DT) carrier protein via the cysteinyl side chain, with a peptide-carrier ratio of 5-8 (A. C...
example 2
Immunological Studies on Minimal Tat Protein Amino Acid Sequences Necessary for Binding to Antibody for Primate-Recognized Epitope II in HIV-1 Tat Protein, Sequence Variations, and Immunological Cross-Reactivities of Antiserums to these Sequences
[0155] Using the same procedures outlined in Example 1, the incidence of amino acid sequence variation for 294 B clade and 56 non-B lade HIV-1 Tat sequences was determined within the Epitope II boundaries of antibody binding in monkeys. The results are reported in Tables VII and VIII. The top lines of the tables contain the consensus sequence. The middle lines contain the percent incidence of amino acids found in greater than 5% of sequences at each position, if multiple. The bottom line shows the total incidence including amino acids occurring in greater than 5% of sequences, if multiple. The amino acid variants at Tat position 42 were antigenically cross-reactive.
TABLE VIIEpitope II- 294 B cladesLys41Gly42Leu43Gly44Ile45Ser46Tyr47Gly48A...
example 3
Development of Human Monoclonal Antibody Treatment for Asymptomatic HIV-1 Infections
[0159] Commercially available antibody humanized mice are immunized with a suitable amount of the Epitope II immunogen: Cys-Gly-Ser-Lys-Gly-Leu-Gly-Ile-Ser-Tyr-Gly-Arg-Lys-amide (SEQ ID NO:34) coupled to diphtheria toxoid carrier protein. Hybridomas are screened on biotin- Ser-Gly-Ser-Gly-Leu-Gly-Ile-Ser-Tyr-Gly-Arg-Lys-OH (SEQ ID NO: 35) on streptavidin-coated plates, and an IgG monoclonal antibody with subnanomolar binding affinity and no binding to complement receptors is selected. Specificity is confirmed on recombinant HIV-1 Tat protein.
[0160] Since the monoclonal antibody is directed to a non-self antigen, a conventional pre-clinical production, purification and safety testing is anticipated. Human monoclonal antibodies have a half-life of 20 days in man vs. the 18 hours half-life of OKT3, a mouse monoclonal antibody which is extensively consumed on internal CD3. Daily doses of 5 mg OKT3 main...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com