Method for diagnosis of thrombotic disorders
a thrombotic disorder and thrombotic disease technology, applied in the field of hematology and blood coagulation disorders, can solve the problems of not including the methodology of testing the two groups of patients described above, and testing cannot be used on plasma from two important patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
1. Test Plasma Samples
[0040] Four and one-half ml of venous blood were collected into a siliconized BD Vacutainer tube containing 0.5 ml of 3.2% sodium citrate. Platelet poor plasma was prepared by centrifugation at 3,000 rpm for 15 minutes at room temperature. Plasma samples were kept frozen in capped plastic tubes at −70° C. to −80° C. until tested.
[0041] Plasma was obtained from the following sources: (1) 39 asymptomatic volunteers from the staff of the Laboratory Service of UCSD Medical Center and the staff of a research laboratory; (2) 21 randomly selected patients receiving oral anticoagulant therapy with warfarin; (3) 29 patients receiving oral anticoagulant activity as determined from a combination of a) a prolonged APTT that failed to correct upon a 1:1 mix with normal plasma, b) increasing apparent coagulant activity with increasing dilution of the plasma in assays for factors VIII, IX, XI, and XII, and c) an abnormal dilute Russell's viper venom t...
example 2
Stand of the Analysis of Test Results
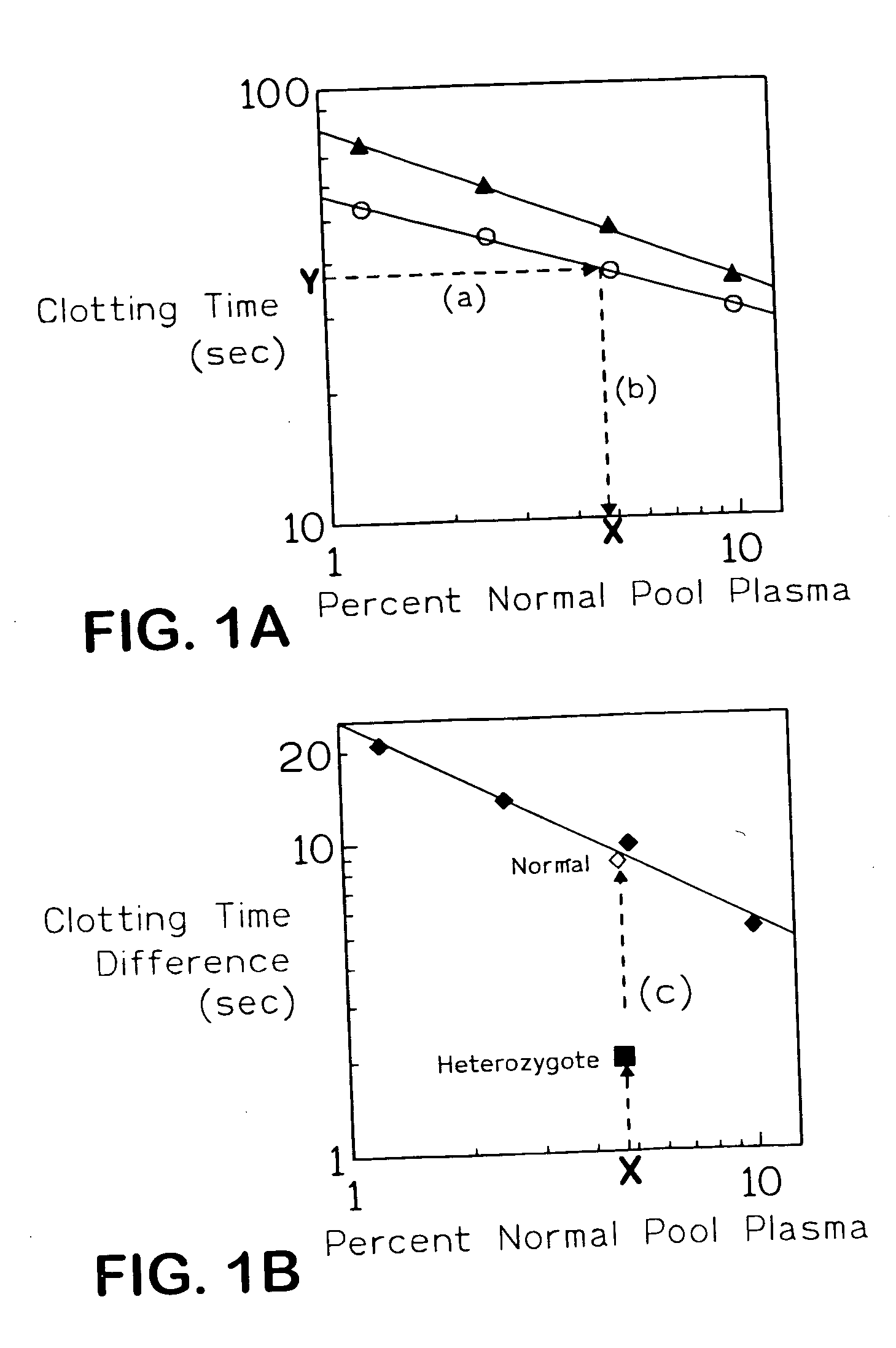
[0047]FIG. 1 shows primary and secondary plots obtained on FV assay with and without APC of dilutions of normal pooled reference plasma. FIG. 1A shows reference curves obtained by plotting the log of clotting times against log of percent concentration of pooled normal reference plasma (primary plot) for the FV assay performed with (▴) and without (o) APC. (Y) a hypothetical clotting time obtained with a test sample which is converted to an equivalent dilution (X) of pooled normal reference plasma via steps (a) and (b). FIG. 1B shows a plot of the log of the difference in the clotting time obtained in the FV assay with and without APC against the log of percent concentration of pooled normal reference plasma. The lines in both plots are linear regression lines of the data. (♦) clotting time differences derived from data of FIG. 1A; (⋄) hypothetical result from a normal subject; (▪) hypothetical result from a heterozygous FY R506Q subject.
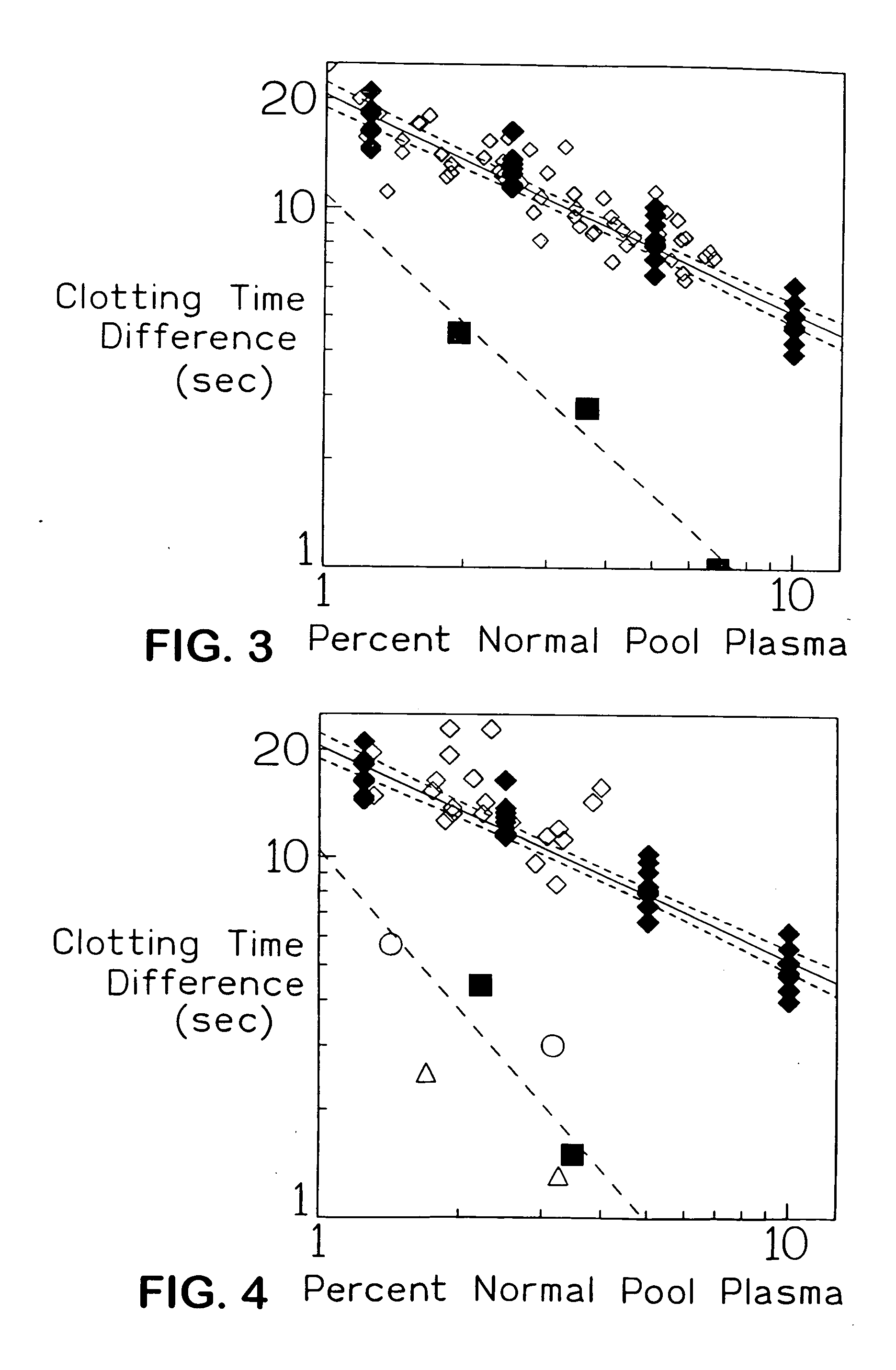
[0048]F...
example 3
Test Results From Control Subjects
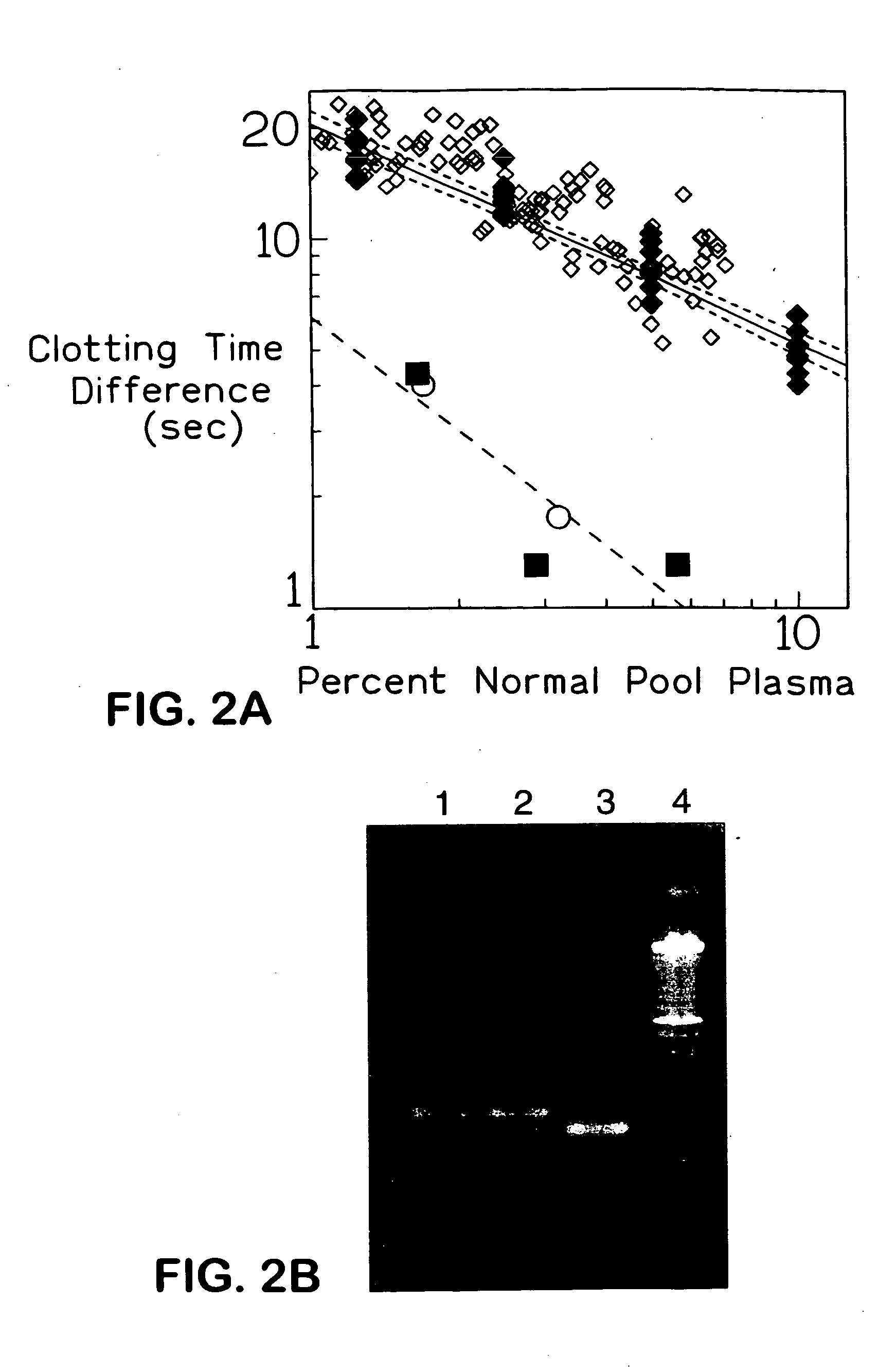
[0053] The clotting time differences obtained on performing FV assays with and without APC on two or three dilutions of each of the plasma samples obtained from 39 control subjects are illustrated in secondary plots FIG. 2A. FIG. 2A) clotting time differences obtained when the FV assay was performed with and without APC for dilutions of plasma from control subjects are plotted against their respective equivalent dilutions of pooled normal reference plasma. (♦) pooled normal reference plasma; (⋄) 37 of the control subjects; (▪, o) two control subjects subsequently discovered to be heterozygotes for FV R506Q. Lower dashed line is regression line of the data obtained with dilutions of plasma from heterozygotes for FV R506Q. The mean regression line of the secondary plots obtained from assays of dilutions of pooled normal reference plasma on 10 different days is indicated by the solid line. The dotted lines delineate the 95% confidence limits of the sl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com